Photo from wikipedia
Supplemental Digital Content is Available in the Text. Objectives: To evaluate the effects of prophylactic piperacillin-tazobactam (PT) on inpatient acute kidney injury (AKI) and fracture-related infection (FRI) in patients with… Click to show full abstract
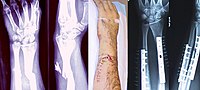
Supplemental Digital Content is Available in the Text. Objectives: To evaluate the effects of prophylactic piperacillin-tazobactam (PT) on inpatient acute kidney injury (AKI) and fracture-related infection (FRI) in patients with open fractures. Setting: The study was conducted at a Level 1 trauma center. Patients: We reviewed 358 Gustilo–Anderson type II and III open fractures at our institution from January 2013 to December 2017. Intervention: Administration of PT (the PT group) or antibiotics other than PT (the historical control group) during the first 48 hours of arrival for open fracture antibiotic prophylaxis. Main Outcome Measurements: The main outcome measurements were rates of inpatient AKI and FRI within six months after definitive fixation. Results: There were 176 patients in the PT group and 182 patients in the historical control group. The PT group had worse American Society of Anesthesiologists class (P = 0.004) and injury severity scores (P < 0.001), a higher average number of debridements before closure/coverage (P = 0.043), and higher rates of gross soil contamination (P = 0.049) and staged procedures (P = 0.008) compared with the historical control group. There was no difference in the rate of AKI between the PT and historical control groups (5.7% vs. 2.7%, P = 0.166) nor when stratified by Gustilo–Anderson fracture classification (type II: 5.8% vs. 3.6%, P = 0.702; type III: 5.6% vs. 2.0%, P = 0.283). There was no significant difference in the rate of FRI between the PT and historical control groups (23.6% vs. 19.6%, P = 0.469). Conclusion: The use of PT in prophylactic antimicrobial treatment in patients with Gustilo–Anderson type II and III open fractures does not increase the rate of AKI or FRI. We believe PT can be used as an effective monotherapy in these patients without an increased risk of renal injury, but future investigations are necessary. Level of Evidence: Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
Journal Title: Journal of Orthopaedic Trauma
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!