Photo from wikipedia
Background and Methods: The present review aims to evaluate the current state-of-the-art dosing regimens of high-dose (HD) and intrathecal methotrexate (MTX) using therapeutic drug monitoring (TDM) to optimize its therapeutic… Click to show full abstract
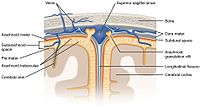
Background and Methods: The present review aims to evaluate the current state-of-the-art dosing regimens of high-dose (HD) and intrathecal methotrexate (MTX) using therapeutic drug monitoring (TDM) to optimize its therapeutic response and minimize associated toxicity, particularly in the central nervous system (CNS). Results: MTX is administered systemically in a HD regimen (>1 g/m2) for the treatment of various hematological neoplasms. HD-MTX treatment becomes complicated by marked interindividual drug elimination variability. TDM is specified to manage this high variability. Approximately 3%–7% of adults with acute lymphoblastic leukemia are diagnosed with CNS involvement, and the incidence of CNS relapse in patients, despite receiving prophylaxis, ranges from 5% to 10%. HD-MTX penetrates the blood–brain barrier and can be administered intrathecally, making this drug an important component of chemotherapy regimens for patients with hematologic malignancies involving the CNS or those at high risk of CNS relapse. Conclusions: The major evidence found was that an MTX area under the curve target between 1000 and 1100 μmol hour−1 L is associated with better clinical outcomes. However, there seems to be a clinical gap in the prospective validation of HD and IT MTX management to optimize clinical outcomes and minimize toxicity, using the relationship between exposure level (area under the curve MTX) and optimal response to MTX, at systemic and CNS exposure.
Journal Title: Therapeutic Drug Monitoring
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!