Photo from wikipedia
Numerous receptor tyrosine kinases and immune receptors activate phospholipase C-gamma (PLC-γ) isozymes at membranes to control diverse cellular processes including phagocytosis, migration, proliferation, and differentiation. The molecular details of this… Click to show full abstract
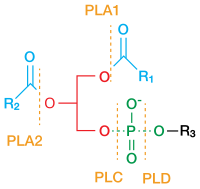
Numerous receptor tyrosine kinases and immune receptors activate phospholipase C-gamma (PLC-γ) isozymes at membranes to control diverse cellular processes including phagocytosis, migration, proliferation, and differentiation. The molecular details of this process are not well understood. Using hydrogen-deuterium exchange mass spectrometry (HDX-MS), we show that PLC-γ1 is relatively inert to lipid vesicles that contain its substrate, PIP2, unless first bound to the kinase domain of the fibroblast growth factor receptor (FGFR1). Exchange occurs throughout PLC-γ1 and is exaggerated in PLC-γ1 containing an oncogenic substitution (D1165H) that allosterically activates the lipase. These data support a model whereby initial complex formation shifts the conformational equilibrium of PLC-γ1 to favor activation. This receptor-induced priming of PLC-γ1 also explains the capacity of a kinase-inactive fragment of FGFR1 to modestly enhance the lipase activity of PLC-γ1 operating on lipid vesicles but not a soluble analog of PIP2 and highlights cooperativity between receptor engagement and membrane proximity. Priming is expected to be greatly enhanced for receptors embedded in membranes and nearly universal for the myriad of receptors and co-receptors that bind the PLC-γ isozymes.
Journal Title: eLife
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!