Photo from wikipedia
We present a detailed study of the kinetic cluster growth process during gelation of weakly attractive colloidal particles by means of experiments on critical Casimir attractive colloidal systems, simulations, and… Click to show full abstract
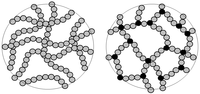
We present a detailed study of the kinetic cluster growth process during gelation of weakly attractive colloidal particles by means of experiments on critical Casimir attractive colloidal systems, simulations, and analytical theory. In the experiments and simulations, we follow the mean coordination number of the particles during the growth of clusters to identify an attractive-strength independent cluster evolution as a function of mean coordination number. We relate this cluster evolution to the kinetic attachment and detachment rates of particles and particle clusters. We find that single-particle detachment dominates in the relevant weak attractive-strength regime, while association rates are almost independent of the cluster size. Using the limit of single-particle dissociation and size-independent association rates, we solve the master kinetic equation of cluster growth analytically to predict power-law cluster mass distributions with exponents -3/2 and -5/2 before and after gelation, respectively, which are consistent with the experimental and simulation data. These results suggest that the observed critical Casimir-induced gelation is a second-order nonequilibrium phase transition (with broken detailed balance). Consistent with this scenario, the size of the largest cluster is observed to diverge with power-law exponent according to three-dimensional percolation on approaching the critical mean coordination number.
Journal Title: Physical review. E
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!