Photo from wikipedia
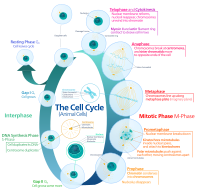
Contraction of the cytokinetic ring during cell division leads to physical partitioning of a eukaryotic cell into two daughter cells. This involves flows of actin filaments and myosin motors in… Click to show full abstract
Contraction of the cytokinetic ring during cell division leads to physical partitioning of a eukaryotic cell into two daughter cells. This involves flows of actin filaments and myosin motors in the growing membrane interface at the midplane of the dividing cell. Assuming boundary driven alignment of the actomyosin filaments at the inner edge of the interface, we explore how the resulting active stresses influence the flow. Using the continuum gel theory framework, we obtain exact axisymmetric solutions of the dynamical equations. These solutions are consistent with experimental observations on closure rate. Using these solutions, we perform linear stability analysis for the contracting ring under nonaxisymmetric deformations. Our analysis shows that few low wave number modes, which are unstable during onset of the constriction, later on become stable when the ring shrinks to smaller radii, which is a generic feature of actomyosin ring closure. Our theory also captures how the effective tension in the ring decreases with its radius, causing significant slowdown in the contraction process at later times.
Journal Title: Physical review letters
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!