Photo from wikipedia
The crystal structures of miconazole {MIC, C18H14Cl4N2O, systematic name (RS)-1-[2-(2,4-dichlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole}, its ethanol monosolvate (C18H14Cl4N2O·C2H5OH) and its hemihydrate (C18H14Cl4N2O·0.5H2O) were compared. A detailed comparison of the molecular conformation of the miconazole… Click to show full abstract
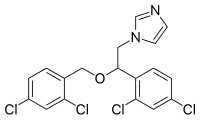
The crystal structures of miconazole {MIC, C18H14Cl4N2O, systematic name (RS)-1-[2-(2,4-dichlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole}, its ethanol monosolvate (C18H14Cl4N2O·C2H5OH) and its hemihydrate (C18H14Cl4N2O·0.5H2O) were compared. A detailed comparison of the molecular conformation of the miconazole molecules showed a structural similarity of the solvate forms, whereas the unsolvated form is related to the gas-phase structure. This suggests that the molecular conformation of miconazole is influenced by solvent molecules. The crystal architectures of the considered solvatomorphs are differentiated by the intermolecular interactions formed by ethanol and water molecules. The structural studies are enriched by Hirshfeld surface and energy framework analysis. The pairwise model energies of the dominant contacts were estimated to be in the range 20-70 kJ mol-1. It is interesting that the contribution of dispersive forces predominates over the electrostatic forces.
Journal Title: Acta crystallographica. Section C, Structural chemistry
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!