Photo from wikipedia
Implementing medical devices into a clinical setting is a complex and lengthy process. Existing models, such as Rogers’ Diffusion of Innovation, try to elucidate this process, however, need to be… Click to show full abstract
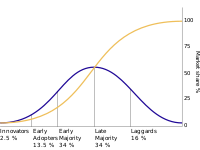
Implementing medical devices into a clinical setting is a complex and lengthy process. Existing models, such as Rogers’ Diffusion of Innovation, try to elucidate this process, however, need to be updated to the evolving healthcare context as fewer than 7% of devices achieve implementation. The aim of this systematic review was to describe the barriers to diffusion in relation to this model to understand why so few technologies are implemented and how to address these challenges to mitigate risk during the translation process. To do this, we searched PubMed, Medline, and Embase databases for studies published between 01/01/1999 – 30/10/2019. Theoretical and application studies were included, and thematic analysis was employed using the Braun and Clarke framework to generate broad themes from the specific concepts described by included studies. A total of 33 articles were eligible for inclusion. Innovation processes constituting an obstacle to diffusion included: technology-specific challenges (8/33), clinical evidence/uncertainty (5/33), regulatory affairs (6/33), health technology assessment (7/33), reimbursement (15/33), and adoption (6/33). The factors that contributed to these themes were identified as being associated to the 11 tenets of Rogers’ Diffusion of Innovation. This allowed the discussion of the identified barriers to medical device diffusion in relation with the Rogers’ model. This analysis enabled the development and proposal of a framework that incorporates considerations within commercialization and translation strategies for these barriers to ultimately facilitate medical technology implementation.
Journal Title: IEEE Access
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!