Photo from wikipedia
To the Editor, Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm and characterized by the Philadelphia chromosome (Ph) with a reciprocal translocation between chromosomes 9q34 and 22q11.2 [1]. The translocation… Click to show full abstract
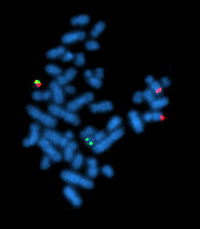
To the Editor, Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm and characterized by the Philadelphia chromosome (Ph) with a reciprocal translocation between chromosomes 9q34 and 22q11.2 [1]. The translocation results in the BCR–ABL1 oncogene, which fuses the 50 sequences of the breakpoint cluster region (BCR) gene on chromosome 22 with 30 sequences of the Abelson (ABL1) gene on chromosome 9 [2]. By understanding of the molecular and cytogenetic abnormal condition, the survival rate of CML patients has been significantly improved by several small-molecule ABL1 kinase inhibitors (imatinib, nilotinib, and dasatinib) [3]. In CML, two typical (e13a2 and e14a2) and a number of atypical BCR–ABL1 transcripts (e1a2, e1a3, e13a3, e14a3, e19a2, e6a2, and e8a2), resulting from BCR on 22q11 and ABL1 on 9q34, have been reported [4, 5]. Adhering to the current National Comprehensive Cancer Network practice guidelines (NCCN, v1.2016), cytogenetics, fluorescence in situ hybridization (FISH), and quantitative reverse-transcription polymerase chain reaction (qRT-PCR) should be a part of the initial workup for CML. We report here a first case of a positive t(9;22)(q34;q11) CML patient with an e9a1 BCR–ABL1 fusion transcript. A 56-year-old male patient presented with a persistently elevated white blood cell (WBC) count and edema of lower extremities in our hospital. His WBC count was 189.28 9 10/L with a differential of 0% myeloblasts, 0% promyelocytes, 5% myelocytes, 8% metamyelocytes, 1.0% eosinophils, 75% neutrophils, 5% basophils, 1% monocytes, and 5% lymphocytes. The levels of hemoglobin and platelet counts were normal. Abdominal ultrasound showed 200 mm splenomegaly. Bone marrow biopsy results indicated a markedly hypercellular marrow with myeloid predominance. Karyotype analysis revealed 46, XY, t (9;22) (q34; q11) [20/20]. (Figure 1a). The reverse-transcription polymerase chain reaction (RT-PCR) specific for p210, p190, and p230 BCR–ABL1 fusion transcripts were performed and failed to detect these BCR– ABL1 transcripts, despite robust amplification of a control gene (E2A). (data not shown). However, FISH confirmed the positive BCR–ABL1 translocation in 68% of bone marrow cells inspected. (Figure 1c). To further identify the BCR–ABL1 breakpoint in this patient, RT-PCR was performed with additional primer sets which covered most of the previously reported uncommon breakpoints [4, 6]. However, both typical and atypical BCR–ABL1 transcripts had not been detected by RT-PCR. Surprisingly, a nearly 1500-bp PCR product with the e9 and a1 primer pair was amplified successfully. (Figure 1b) By DNA sequencing of the PCR product, the e9a1 fusion mRNA sequence was confirmed. (Figure 1d). To our knowledge, no report on the breakpoint in BCR exon 9 and ABL1 exon 1 in CML patients has previously been published. Then, he was treated with Gleevec (imatinib). Fortunately, our patient appeared to have a good response to imatinib and had a rather typical clinical progression, although poor tolerance of it. At first, he was treated with imatinib for a total of 50 days (400 mg/day) followed by 7 days discontinued because of the thrombocytopenia. Subsequently, he resumed the drug without interruption. The follow-up routine blood test demonstrated a markedly reduced WBC, and a normal size of spleen indicating a good clinical response. Three months later, a follow-up BCR–ABL1 FISH confirmed a partial cytogenetic response with a markedly reduced BCR–ABL1 fusion rate of 41%, because our routine qRT-PCR assay was unable to detect this rare fusion. According to the current NCCN practice guidelines (v1.2016), the examinations of cytogenetics, FISH, and RT-PCR are indispensible for CML patients at diagnosis. Although all of them mainly detect the Ph chromosome and the BCR–ABL1 fusion abnormality, each of them can provide unique information. The karyotype analysis is a cytogenetic test, which not only shows the morphologic review, but also identifies any abnormalities other than Ph chromosome at initial workup [7]. QRT-PCR of BCR–ABL1 RNA is a sensitive and necessary laboratory technique for monitoring the efficacy of tyrosine kinase inhibitor (TKI) therapy and quantitatively assessing the minimal residual disease (MRD), while RT-PCR assays are limited in the unusual breakpoints detected based on
Journal Title: International Journal of Laboratory Hematology
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!