Photo from wikipedia
Dear Editor, There is a need to assess adverse events of booster immunizations in patients with autoimmune blistering disorders (AIBDs). In this crosssectional study, Englishspeaking patients with AIBDs aged ≥18… Click to show full abstract
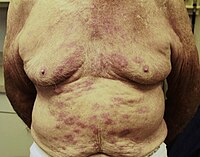
Dear Editor, There is a need to assess adverse events of booster immunizations in patients with autoimmune blistering disorders (AIBDs). In this crosssectional study, Englishspeaking patients with AIBDs aged ≥18 years, who were recruited from the database of the International Pemphigus and Pemphigoid Foundation, were asked to complete a COVID19 vaccinationrelated webbased survey between 1 April 2022 and 30 April 2022. Only patients who received a COVID19 booster vaccine (i.e. third dose in the case of previous PfizerBioNTech, Moderna or AstraZeneca vaccine OR second dose in the case of previous Johnson & Johnson vaccine) were included. Electronic informed consent was obtained, and the questionnaire was completed anonymously. The study was granted an exemption by the Institutional Review Board of the University of Southern California. Valid questionnaires were collected from a total of 495 patients [378 women and 117 men, aged 18– 94 years (median 66); 239 (48.3%) pemphigus vulgaris, 114 (23.0%) mucous membrane pemphigoid, 96 (19.4%) bullous pemphigoid, 29 (5.9%) pemphigus foliaceus and 17 (3.4%) other AIBDs]. The types of COVID19 booster vaccines were PfizerBioNTech (55.6%; n = 275), Moderna (41.6%; n = 206), Johnson & Johnson (1.4%; n = 7), AstraZeneca (1.0%; n = 5) or other vaccine type (0.4%; n = 2). 189 (38.2%) patients reported not being on any therapy for their AIBD at the time of the booster, whereas others mentioned concurrent treatment with topical corticosteroids (26.1%; n = 129), oral corticosteroids (20.6%; n = 102), mycophenolate mofetil (13.3%; n = 66), rituximab (within 1 year) (12.9%; n = 64), doxycycline (7.5%; n = 37), dapsone (6.5%; n = 32), azathioprine (3.6%; n = 18), methotrexate (3.2%; n = 16), intravenous immunoglobulins (3.2%; n = 16), cyclosporine (0.4%; n = 2), cyclophosphamide (0.2%; n = 1), immunoadsorption/plasmapheresis (0.2%; n = 1) and/ or other therapy (7.7%; n = 38). Out of 314 responders, 31 (9.9%) intentionally reduced or discontinued therapy for their AIBD in anticipation of the booster vaccine. A total of 206 (41.6%) patients experienced COVID19 booster vaccineassociated adverse events including selfperceived flare or worsening of their AIBD (17.0%; n = 84), the latter occurring within less than a week to more than 1 month after the injection (Table 1). Thirtyseven (44.0%) patients stated that this flare/worsening was confirmed by a dermatologist. Four out of 31 (13.0%) patients who modified their treatment prior to the booster were among those with disease aggravation. Fiftyfour (64.3%) patients required additional treatment for their disease exacerbation, which led to its control in 37 (68.5%) cases, and only 4 (7.4%) patients had to be hospitalized for this reason. Thirtyseven (7.5%), 50 (10.1%) and 19 (3.8%) patients noticed a disease flare/ worsening after their previous first, second and both the first and second COVID19 vaccine doses, respectively, with 38 (55.9%), 16 (23.5%) and 14 (20.6%) of them reporting a milder, similar or worse postbooster course compared with the initial dose(s), respectively. Overall, COVID19 booster vaccines basically do not seem to be associated with an increased risk of adverse events when compared with the primary vaccination series.1 This also applies to AIBD flare/worsening, which was mostly reported as being even comparatively milder after Received: 7 June 2022 | Accepted: 27 July 2022
Journal Title: Journal of the European Academy of Dermatology and Venereology
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!