Photo from wikipedia
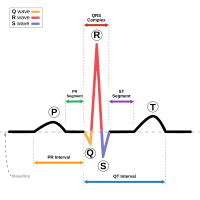
INTRODUCTION More than 40,000 children undergo surgical interventions annually for the treatment of congenital heart defects. Intraoperative and postoperative vital sign monitoring is a cornerstone of pediatric care. METHODS A… Click to show full abstract
INTRODUCTION More than 40,000 children undergo surgical interventions annually for the treatment of congenital heart defects. Intraoperative and postoperative vital sign monitoring is a cornerstone of pediatric care. METHODS A single-arm prospective observational study was performed. Pediatric patients undergoing a procedure with a planned admission to the Cardiac Intensive Care Unit at Lurie Children's Hospital (Chicago, IL) were eligible for enrollment. Participant vital signs were monitored using standard equipment and an FDA-cleared experimental device (ANNE® ) consisting of a wireless patch positioned at the suprasternal notch and index finger or foot. The primary goal of the study was to assess real-world feasibility of wireless sensors in pediatric patients with congenital cardiac defects. RESULTS A total of 13 patients were enrolled, ranging in age from 4 months to 16 years with a median age of 4 years. Overall, 54% (n = 7) were female and the most common anomaly in the cohort was an atrial septal defect (n = 6). The mean admission length was 3 days (range 2-6), resulting in more than 1000 h of vital sign monitoring (⟩60,000 data points). Bland-Altman plots were generated for heart rate and respiratory rate to assess beat-to-beast differences between the standard equipment and the experimental sensors. CONCLUSIONS Novel, wireless, flexible sensors demonstrated comparable performance to standard monitoring equipment in a cohort of pediatric patients with congenital cardiac heart defects undergoing surgery.
Journal Title: Paediatric anaesthesia
Year Published: 2023
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!