Photo from wikipedia
Two mild approaches generate radical intermediates from masked aldehydes Radical intermediates are molecules that form transiently during a reaction and bear an unpaired electron. Molecules containing an unpaired electron are… Click to show full abstract
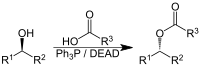
Two mild approaches generate radical intermediates from masked aldehydes Radical intermediates are molecules that form transiently during a reaction and bear an unpaired electron. Molecules containing an unpaired electron are often viewed as challenging to access under mild conditions and accordingly have been underexploited as synthetic intermediates. Chemists are now developing catalytic methods to facilitate access to versatile radical intermediates (1). Despite recent advances, some types of potentially useful radical intermediates remain difficult to engage. On page 225 of this issue, Wang et al. (2) report a new approach for accessing elusive ketyl radicals. They leverage an in situ masking strategy of alkyl aldehydes and unlock opportunities for electron-transfer or atom-transfer catalysis.
Journal Title: Science
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!