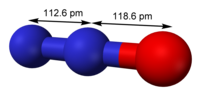
Photo from wikipedia
Twenty-five percent inhaled nitrous oxide improves symptoms of treatment-resistant major depression with fewer adverse effects than the 50% concentration. Laughing gas improves depression About one-third of individuals suffering from depression… Click to show full abstract
Twenty-five percent inhaled nitrous oxide improves symptoms of treatment-resistant major depression with fewer adverse effects than the 50% concentration. Laughing gas improves depression About one-third of individuals suffering from depression are at risk for treatment resistance. Whereas inhaled 50% nitrous oxide has early antidepressant effects on individuals with treatment-resistant major depression (TRMD), adverse effects can occur at this concentration. In this phase 2 clinical trial, Nagele et al. studied the effects of a single 1-hour treatment with 25% nitrous oxide on depression symptoms in those with TRMD, finding that this lower concentration had comparable efficacy to 50% nitrous oxide over several weeks but was associated with significantly fewer adverse effects. These results highlight that lower concentrations of nitrous oxide may be a useful treatment for TRMD. Nitrous oxide at 50% inhaled concentration has been shown to improve depressive symptoms in patients with treatment-resistant major depression (TRMD). Whether a lower concentration of 25% nitrous oxide provides similar efficacy and persistence of antidepressant effects while reducing the risk of adverse side effects is unknown. In this phase 2 clinical trial (NCT03283670), 24 patients with severe TRMD were randomly assigned in a crossover fashion to three treatments consisting of a single 1-hour inhalation with (i) 50% nitrous oxide, (ii) 25% nitrous oxide, or (iii) placebo (air/oxygen). The primary outcome was the change on the Hamilton Depression Rating Scale (HDRS-21). Whereas nitrous oxide significantly improved depressive symptoms versus placebo (P = 0.01), there was no difference between 25 and 50% nitrous oxide (P = 0.58). The estimated differences between 25% and placebo were −0.75 points on the HDRS-21 at 2 hours (P = 0.73), −1.41 points at 24 hours (P = 0.52), −4.35 points at week 1 (P = 0.05), and −5.19 points at week 2 (P = 0.02), and the estimated differences between 50% and placebo were −0.87 points at 2 hours (P = 0.69), −1.93 points at 24 hours (P = 0.37), −2.44 points at week 1 (P = 0.25), and −7.00 points at week 2 (P = 0.001). Adverse events declined substantially with dose (P < 0.001). These results suggest that 25% nitrous oxide has comparable efficacy to 50% nitrous oxide in improving TRMD but with a markedly lower rate of adverse effects.
Journal Title: Science Translational Medicine
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!