Photo from wikipedia
Zika virus (ZIKV) is the mosquito-borne enveloped flavivirus that causes microcephaly in neonates. While real-time imaging plays a critical role in dissecting viral biology, no fluorescent, genetically engineered ZIKV for… Click to show full abstract
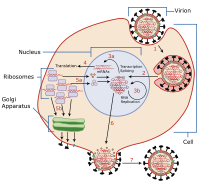
Zika virus (ZIKV) is the mosquito-borne enveloped flavivirus that causes microcephaly in neonates. While real-time imaging plays a critical role in dissecting viral biology, no fluorescent, genetically engineered ZIKV for single-particle tracking is currently available. ABSTRACT Real-time imaging of viruses in living cells considerably facilitates the study of virus-host interactions. However, generating a fluorescently labeled recombinant virus is challenging, especially for Zika virus (ZIKV), which causes microcephaly in neonates. The monocistronic nature of the ZIKV genome represents a major challenge for generating a replication-competent genetically engineered ZIKV suitable for real-time imaging. Here, we generated a fluorescent ZIKV by introducing the biarsenical tetracysteine (TC) tag system. After separately inserting the TC tag at six sites in the capsid protein, we found that only when we inserted the TC tag at the site of amino acids 27/28 (AA27/28, or TC27) could the genetically engineered ZIKV be rescued. Importantly, the TC27 ZIKV is characterized as replication and infection competent. After labeling the TC tag with the fluorescent biarsenical reagents, we visualized the dynamic nuclear import behavior of the capsid protein. In addition, using the single-particle tracking technology, we acquired real-time imaging evidence that ZIKV moved along the cellular filopodia and entered into the cytoplasm via endocytosis. Thus, we provide a feasible strategy to generate a replication-competent TC-tagged ZIKV for real-time imaging, which should greatly facilitate the study of ZIKV-host interactions in living cells. IMPORTANCE Zika virus (ZIKV) is the mosquito-borne enveloped flavivirus that causes microcephaly in neonates. While real-time imaging plays a critical role in dissecting viral biology, no fluorescent, genetically engineered ZIKV for single-particle tracking is currently available. Here, we generated a replication-competent genetically engineered ZIKV by introducing the tetracysteine (TC) tag into its capsid protein. After labeling the TC tag with the fluorescent biarsenical reagents, we visualized the nuclear import behavior of the capsid protein and the endocytosis process of single ZIKV particle. Taken together, these results demonstrate a fluorescent labeling strategy to track the ZIKV-host interactions at both the protein level and the viral particle level. Our replication-competent TC27 ZIKV should open an avenue to study the ZIKV-host interactions and may provide applications for antiviral screening.
Journal Title: Journal of Virology
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!