Photo from wikipedia
Many antibiotics were originally discovered from microbes. However, in recent decades, resistance to current treatments has risen, while novel antibiotic discovery has become increasingly challenging. ABSTRACT Bacterial natural products have… Click to show full abstract
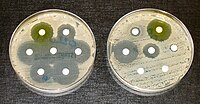
Many antibiotics were originally discovered from microbes. However, in recent decades, resistance to current treatments has risen, while novel antibiotic discovery has become increasingly challenging. ABSTRACT Bacterial natural products have historically been a deep source of new medicines, but their slowed discovery in recent decades has put a premium on developing strategies that enhance the likelihood of capturing novel compounds. Here, we used a straightforward approach that capitalizes on the interactive ecology of “rare” actinomycetes. Specifically, we screened for interactions that triggered the production of antimicrobials that inhibited the growth of a bacterial strain with exceptionally diverse natural antimicrobial resistance. This strategy led to the discovery of a family of antimicrobials we term the dynaplanins. Heterologous expression enabled identification of the dynaplanin biosynthetic gene cluster, which was missed by typical algorithms for natural product gene cluster detection. Genome sequencing of partially resistant mutants revealed a 2-oxo acid dehydrogenase E2 subunit as the likely molecular target of the dynaplanins, and this finding was supported by computational modeling of the dynaplanin scaffold within the active site of this enzyme. Thus, this simple strategy, which leverages microbial interactions and natural antibiotic resistance, can enable discovery of molecules with unique antimicrobial activity. In addition, these results indicate that primary metabolism may be a direct target for inhibition via chemical interference in competitive microbial interactions. IMPORTANCE Many antibiotics were originally discovered from microbes. However, in recent decades, resistance to current treatments has risen, while novel antibiotic discovery has become increasingly challenging. Thus, there is a need to develop new strategies to find novel antimicrobials. Here, we incorporated three levels of innovation into a single, simple discovery pipeline: focusing on understudied bacteria with a high potential for producing antibiotics, growing these bacteria in binary microbial interactions, and screening for activity against a multidrug-resistant bacterium. This led us to discover a family of antimicrobials that we call the dynaplanins, which are synthesized by genes that were not detected by typical prediction algorithms. We found that dynaplanins likely block the function of one of three related enzymes called 2-oxo acid dehydrogenases, which are vital to cellular metabolism. Overall, our strategy based on bacterial competition led to discovery of a novel antibiotic that inhibits the ability to metabolize nutrients.
Journal Title: mBio
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!