Photo from wikipedia
ABSTRACT Candida biofilms resist the effects of available antifungal therapies. Prior studies with Candida albicans biofilms show that an extracellular matrix mannan-glucan complex (MGCx) contributes to antifungal sequestration, leading to… Click to show full abstract
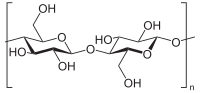
ABSTRACT Candida biofilms resist the effects of available antifungal therapies. Prior studies with Candida albicans biofilms show that an extracellular matrix mannan-glucan complex (MGCx) contributes to antifungal sequestration, leading to drug resistance. Here we implement biochemical, pharmacological, and genetic approaches to explore a similar mechanism of resistance for the three most common clinically encountered non-albicans Candida species (NAC). Our findings reveal that each Candida species biofilm synthesizes a mannan-glucan complex and that the antifungal-protective function of this complex is conserved. Structural similarities extended primarily to the polysaccharide backbone (α-1,6-mannan and β-1,6-glucan). Surprisingly, biochemical analysis uncovered stark differences in the branching side chains of the MGCx among the species. Consistent with the structural analysis, similarities in the genetic control of MGCx production for each Candida species also appeared limited to the synthesis of the polysaccharide backbone. Each species appears to employ a unique subset of modification enzymes for MGCx synthesis, likely accounting for the observed side chain diversity. Our results argue for the conservation of matrix function among Candida spp. While biogenesis is preserved at the level of the mannan-glucan complex backbone, divergence emerges for construction of branching side chains. Thus, the MGCx backbone represents an ideal drug target for effective pan-Candida species biofilm therapy. IMPORTANCE Candida species, the most common fungal pathogens, frequently grow as a biofilm. These adherent communities tolerate extremely high concentrations of antifungal agents, due in large part, to a protective extracellular matrix. The present studies define the structural, functional, and genetic similarities and differences in the biofilm matrix from the four most common Candida species. Each species synthesizes an extracellular mannan-glucan complex (MGCx) which contributes to sequestration of antifungal drug, shielding the fungus from this external assault. Synthesis of a common polysaccharide backbone appears conserved. However, subtle structural differences in the branching side chains likely rely upon unique modification enzymes, which are species specific. Our findings identify MGCx backbone synthesis as a potential pan-Candida biofilm therapeutic target. Candida species, the most common fungal pathogens, frequently grow as a biofilm. These adherent communities tolerate extremely high concentrations of antifungal agents, due in large part, to a protective extracellular matrix. The present studies define the structural, functional, and genetic similarities and differences in the biofilm matrix from the four most common Candida species. Each species synthesizes an extracellular mannan-glucan complex (MGCx) which contributes to sequestration of antifungal drug, shielding the fungus from this external assault. Synthesis of a common polysaccharide backbone appears conserved. However, subtle structural differences in the branching side chains likely rely upon unique modification enzymes, which are species specific. Our findings identify MGCx backbone synthesis as a potential pan-Candida biofilm therapeutic target.
Journal Title: mBio
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!