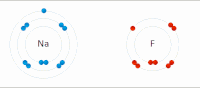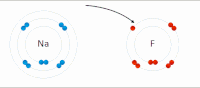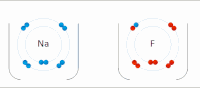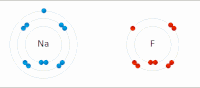
Photo from wikipedia
The electron structure of Li2MnSiO4 and Li2FeSiO4 in a layered orthorhombic crystal structure of Pmn21 is studied by the electron density functional method. Using the analysis of the density of… Click to show full abstract
The electron structure of Li2MnSiO4 and Li2FeSiO4 in a layered orthorhombic crystal structure of Pmn21 is studied by the electron density functional method. Using the analysis of the density of crystal orbital Hamilton populations (COHPs), the features of chemical bond formation in these substances are studied. Anisotropy of the chemical bond of Mn with oxygen atoms is observed for Li2MnSiO4 with the complete extraction of lithium atoms from the structure. The formation of anisotropy of the chemical bond can indicate that Mn is trying to change the coordination and the beginning of the restructuring of the compound structure and its reduced stability.
Journal Title: Glass Physics and Chemistry
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!