Photo from wikipedia
Objectives Systemic lupus erythematosus (SLE) is a chronic autoimmune disease of considerable genetic predisposition. Genome-wide association studies have identified tens of common variants for SLE. However, the majority of them… Click to show full abstract
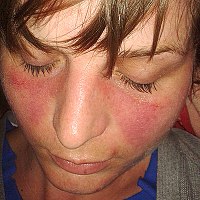
Objectives Systemic lupus erythematosus (SLE) is a chronic autoimmune disease of considerable genetic predisposition. Genome-wide association studies have identified tens of common variants for SLE. However, the majority of them reside in non-coding sequences. The contributions of coding variants have not yet been systematically evaluated. Methods We performed a large-scale exome-wide study in 5004 SLE cases and 8179 healthy controls in a Han Chinese population using a custom exome array, and then genotyped 32 variants with suggestive evidence in an independent cohort of 13 246 samples. We further explored the regulatory effect of one novel non-coding single nucleotide polymorphism (SNP) in ex vivo experiments. Results We discovered four novel SLE gene regions (LCT, TPCN2, AHNAK2 and TNFRSF13B) encompassing three novel missense variants (XP_016859577.1:p.Asn1639Ser, XP_016859577.1:p.Val219Phe and XP_005267356.1:p.Thr4664Ala) and two non-coding variants (rs10750836 and rs4792801) with genome-wide significance (pmeta <5.00×10−8). These variants are enriched in several chromatin states of primary B cells. The novel intergenic variant rs10750836 exhibited an expression quantitative trait locus effect on the TPCN2 gene in immune cells. Clones containing this novel SNP exhibited gene promoter activity for TPCN2 (P=1.38×10−3) whose expression level was reduced significantly in patients with SLE (P<2.53×10−2) and was suggested to be further modulated by rs10750836 in CD19+ B cells (P=7.57×10−5) in ex vivo experiments. Conclusions This study identified three novel coding variants and four new susceptibility gene regions for SLE. The results provide insights into the biological mechanism of SLE.
Journal Title: Annals of the Rheumatic Diseases
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!