Photo from wikipedia
Introduction There is limited evidence on the efficacy of using spirometry routinely in paediatric practice for improving outcomes. Objective To determine whether the routine use of spirometry alters clinical decisions… Click to show full abstract
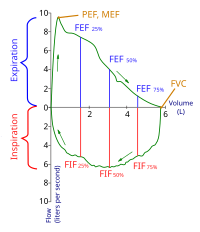
Introduction There is limited evidence on the efficacy of using spirometry routinely in paediatric practice for improving outcomes. Objective To determine whether the routine use of spirometry alters clinical decisions and patient-related outcome measures for children managed by respiratory paediatricians. Methods We undertook a parallel open-label randomised controlled trial involving children (aged 4–18 years) able to perform spirometry in a specialist children’s hospital in Australia. Children were randomised to either routine use of spirometry (intervention) or clinical review without use of spirometry (control) for one clinic visit. The primary outcomes were the (a) proportion of children with ‘any change in clinical decisions’ and (b) ‘change score’ in clinical decisions. Secondary outcomes were change in patient-related outcome measures assessed by State–Trait Anxiety Inventory (STAI) and Parent-Proxy QoL questionnaire for paediatric chronic cough (PC-QoL). Results Of 136 eligible children, 106 were randomised. Compared with controls, the intervention group had significantly higher proportion of children with ‘any change in clinical decisions’ (n=54/54 (100%) vs n=34/52 (65.4%), p<0.001) and higher clinical decision ‘change score’ (median=2 (IQR 1–4) vs 1 (0–2), p<0.001). Also, improvement was significantly greater in the intervention group for overall STAI score (median=−5 (IQR −10 to –2) vs −2.5 (−8.5, 0), p=0.021) and PC-QoL social domain (median=3 (IQR 0 to 5) vs 0 (−1, 1), p=0.017). Conclusion The routine use of spirometry in children evaluated for respiratory issues at clinical outpatient review is beneficial for optimising clinical management and improving parent psychosocial well-being. Registration Australia and New Zealand Clinical Trials Registry ACTRN12619001686190
Journal Title: BMJ Open Respiratory Research
Year Published: 2023
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!