Photo from wikipedia
Cerebral venous thrombosis (CVT) is a unique cause of stroke. Populationbased studies demonstrate an incidence of clinically significant CVT of 1.3–1.5 per 100 000 per year. 2 This may be… Click to show full abstract
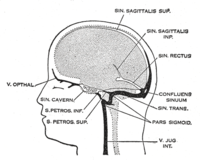
Cerebral venous thrombosis (CVT) is a unique cause of stroke. Populationbased studies demonstrate an incidence of clinically significant CVT of 1.3–1.5 per 100 000 per year. 2 This may be an underestimated incidence, as these studies were not based on review of imaging and the often benign nature of this entity may lead to underdiagnoses. In the JNIS paper published by Cleaver et al, the authors describe their experience with endovascular treatment (EVT) in patients with cerebral venous sinus thrombosis in patients following COVID19 vaccination. CVTrelated stroke primarily affects young adult and middleaged patients. 5 Although initially blamed for having a 50% mortality based on autopsy series, more recent prospective multinational studies (International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT)) have demonstrated a mortality rate of 8% in a cohort of 624 patients. A total of 75% of these patients were female and 13% had a poor outcome (death or dependence). The baseline risk factors for poor outcome included age >37 years (HR=2.0), male sex (HR=1.6), coma (HR=2.7), mental status disorder (HR=2.0), hemorrhage on admission computed tomography (CT) scan (HR=1.9), thrombosis of the deep cerebral venous system (HR=2.9), central nervous system infection (HR=3.3), and cancer (HR=2.9). Only 8% of these patients were >65 years of age, and had a poorer prognosis. Female predilection was lost in the elderly age group, with both sexes affected almost equally. Some 46% of these patients demonstrated infarct on CT/magnetic resonance imaging (MRI) while 39% of these patients demonstrated hemorrhage. Traditionally, all major guidelines have recommended anticoagulation with heparin – preferentially low molecular weight heparin – as the standard treatment for CVT, regardless of the presence of an intracerebral hemorrhage (ICH). Heparin treatment is supposed to prevent growth or embolization of the existing thrombus, and facilitate recanalization. Patients with cerebral sinus thrombosis treated with anticoagulants (low molecular weight heparin followed by oral anticoagulation) had a favorable outcome more often than controls, although the difference was not statistically significant. Hypercoagulability and CVT after COVID19 infection and vaccination has been described, especially after vaccination with the adenovirus vector vaccine ChAdOx1 (Oxford/AstraZeneca), which is associated with very low platelets, antiplatelet factor 4 antibodies and elevated ddimer, leading to the designation of vaccineinduced thrombotic thrombocytopenia (VITT). In a study of 95 patients with CVT, 70 of whom had VITT and 25 did not, primary outcome of death or dependency occurred more frequently in patients with VITTassociated CVT (47%) compared with the nonVITT control group (16%; P=0·0061). The median age of the VITT group (47 years) was lower than in the nonVITT group (57 years; P=0·0045). Patients with VITTassociated CVT had more thrombosed intracranial veins (three) than nonVITT patients (two) and more frequently had extracranial thrombosis (44%) compared with nonVITT patients (4%; P=0·0003). The adverse outcomes were less frequent in patients with VITT who received nonheparin anticoagulants (18/50 (36%) patients) compared with those who did not (15/20 (75%) patients; P=0·0031), and in those who received intravenous immunoglobulin (22/55 (40%) patients) compared with those who did not (11/15 (73%) patients; P=0·022). Treatment guidelines advocate not using heparin in patients with suspected VITT; instead treatment is focused on intravenous immunoglobulin and nonheparin anticoagulation. EVT is advocated in patients with COVID19 infectionrelated emergent large vessel occlusion (ELVO) and to treat patients with CVT. EVT for CVT aims to achieve rapid recanalization of the cerebral venous sinuses through local mechanical thrombectomy, thrombolytic drug administration, or a combination of both. These methods result in more rapid recanalization, by delivering the drug where needed, downstream from the cerebral venous infarcts which may be hemorrhagic, and increasing the surface area of the thrombus exposed to thrombolytics. This is usually advocated when clinical neurologic deterioration occurs despite anticoagulation treatment. The European Stroke Organisation guidelines, however, do not include or provide a recommendation on EVT for CVT, whereas the American Heart Association guideline recommended considering EVT only in patients who deteriorate despite anticoagulant treatment. In a systematic review of mechanical thrombectomy in CVT, of 185 cases in 42 studies, 156 (84%) of patients had a good outcome and 22 died (12%). Some 10% of patients demonstrated new or increased periprocedural hemorrhage with a failure rate of 5%. The TOACT (Thrombolysis or Anticoagulation for Cerebral Venous Thrombosis) trial was designed to test the hypothesis that EVT in addition to standard medical care improves the clinical outcome of patients with severe CVT compared with standard medical care alone. Of 67 enrolled and randomized patients, 33 (49%) were randomized to EVT and standard medical therapy (intervention group) and 34 (51%) were randomized to receive guidelinebased standard medical care (control group). The intervention consisted of mechanical thrombectomy, pharmacological thrombolysis, or a combination of both. The interventional treatment was made at the discretion of the interventionalist, although the maximum doses of pharmacological agents were specified in the trial protocol. Continuous infusion with a thrombolytic drug in the affected sinus was allowed after the initial procedure for a maximum of 72 hours. For mechanical thrombectomy, the use of devices was allowed at the discretion of the interventionalist. At the 12month followup, 22 intervention patients (67%) had a modified Rankin score (mRS) of 0 to 1 compared with 23 control patients (68%) (relative risk ratio 0.99). EVT with standard medical care did not appear to improve functional outcome of patients with CVT, although mortality was not statistically significantly higher in the EVT group (12% vs 3%; P=0.20). The frequency of symptomatic ICH was also not statistically Department of Radiology and Biomedical Imaging, Yale University, New Haven, Connecticut, USA Department of Radiology, Massachusetts General Hospital, Boston, Massachusetts, USA
Journal Title: Journal of NeuroInterventional Surgery
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!