Photo from wikipedia
Inhibition of CDK4/6 has emerged as an effective cancer therapy, but the underlying mechanism remains obscure. We have addressed this question in mantle cell lymphoma (MCL), a Non-Hodgkin9s B cell… Click to show full abstract
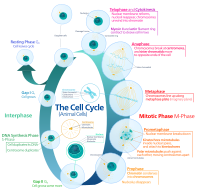
Inhibition of CDK4/6 has emerged as an effective cancer therapy, but the underlying mechanism remains obscure. We have addressed this question in mantle cell lymphoma (MCL), a Non-Hodgkin9s B cell lymphoma that remains incurable due to the development of drug resistance. As dysregulation of CDK4 (CDK6 is silenced) and cyclin D1 underlies unrestrained proliferation of MCL cells, targeting CDK4 also represents a rational approach to MCL therapy. Previously we have demonstrated in preclinical studies that sustained inhibition of CDK4 with palbociclib led to prolonged early G1 arrest (pG1) that both prevented proliferation of MCL cells and reprogrammed them for killing by diverse agents. On this basis, we hypothesized that CDK4 inhibition may prolong and deepen the clinical response to its partner drug in combination therapy. Indeed, in a phase I clinical trial of palbociclib plus ibrutinib (BTK inhibitor) in recurrent MCL (n=28), complete response was observed in 43% of patients as compared with 23% in response to ibrutinib alone. Moreover, the responses were rapid and durable; only 4 of the 18 responding-patients have progressed in the 3.5 years since the trial opened. To address how CDK4 inhibition enhances therapy vulnerability, in a separate phase I clinical trial palbociclib was administered to MCL patients for 12 days to induce pG1; bortezomib was given in pG1 and again after synchronous S phase reentry (pG1-S). At the optimal dose combination only one of 6 patients progressed and complete remission in one patient continues into its 6th year. Longitudinal integrative RNA and exome-sequencing (WTS/WES) of MCL cells isolated from lymph node biopsies at baseline, in pG1 (day 8) and in pG1-S (day 21) of individual patients showed that inhibition of CDK4 induced pG1 in all patients initially, regardless of the subsequent clinical response. However, induction of pG1 led to a striking difference in cellular gene expression in pG1 (day 8/day 1) in MCL cells of responders (R) and non-responders (NR). There were 2041 genes upregulated and 1483 genes downregulated 1.5-fold or greater in R (N=4, EdgeR, FDR 0.05), but only 39 genes upregulated and 401 genes dowregulated in NR (N=3). Moreover, 5 genes were down regulated in R and upregulated in NR. Gene set enrichment analysis together with loss and gain of function studies revealed that among them, loss of IRF4 in R led to de-repression of IRF7 and induction of the interferon response. This in turn resulted in TRAIL mediated apoptosis due to repression of CYTIP downstream of IRF4 repression. In summary, by longitudinal functional genomics of purified MCL cells and functional studies, we have discovered that CDK4 inhibition reprograms MCL for therapy vulnerability through induction of anti-tumor IFN response, and identified CYTIP and IRF4 as biomarkers that discriminate sensitivity from resistance to therapeutic targeting of CDK4. Citation Format: Maurizio Di Liberto, Peter Martin, Xiangao Huang, Priyanka Vijay, David Chiron, Scott Ely, Christopher Mason, Olivier Elemento, John Leonard, Selina Chen-Kiang. Repression of IRF4 and CYTIP unleashes anti-tumor interferon response in CDK4 inhibitor therapy in mantle cell lymphoma [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2018; 2018 Apr 14-18; Chicago, IL. Philadelphia (PA): AACR; Cancer Res 2018;78(13 Suppl):Abstract nr 1523.
Journal Title: Cancer Research
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!