Photo from wikipedia
April 23, 2019 2083 Sripal Bangalore, MD, MHA In Response: Romaguera et al raise several important points about our meta-analysis. First, it is true that the Orsiro stent platform, unlike… Click to show full abstract
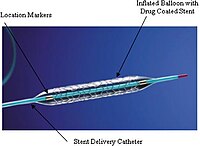
April 23, 2019 2083 Sripal Bangalore, MD, MHA In Response: Romaguera et al raise several important points about our meta-analysis. First, it is true that the Orsiro stent platform, unlike the other 2 ultrathin strut stents compared in our meta-analysis, has a thicker strut for larger stent sizes (≥3.5 mm). However, the mean reference vessel diameters in these trials varied from 2.6 to 3.2 mm, with the majority of trials having a mean reference vessel diameter of 2.7 mm with a SD of 0.4 mm, suggesting that larger stents (≥3.5 mm) were likely used in a minority of patients. Moreover, stratified analysis based on small versus large vessel size in these trials showed no significant interaction with treatment effect.1 Second, the Nobori stent was included in our analysis because the intent was to compare ultrathin stents with thicker secondgeneration drug-eluting stents. Sensitivity analyses excluding this trial did not materially change the results of the analyses.2 Third, the meriT-V trial (BioMime Vs. Xience Randomised Control Clinical Study) contributes <2% of the weight of the analyses for any of the end points.2 Exclusion of this trial does not materially change the results of the analyses. Finally, the authors state that Orsiro increased stent thrombosis 9-fold in comparison with Resolute Onyx in the Bionyx trial (Bioresorbable Polymer ORSIRO Versus Durable Polymer RESOLUTE ONYX Stents). Although, 9-fold seems like an alarming number, at 1-year follow-up, definite or probable stent thrombosis was seen in 1 patient (0.08%) in the Onyx group and 9 patients in the Orsiro group (0.72%).3 The stent thrombosis rate in the Onyx group is out of line with the cumulative data on 1-year stent thrombosis with second-generation drug-eluting stents and could be a chance finding.4 Moreover, these numbers let us calculate that just 1 additional stent thrombosis in the Onyx group would render the results to be statistically nonsignificant (P>0.05). Moreover, the 2-year results of the BIOFLOW-V trial (Safety and Effectiveness of the Orsiro Sirolimus Eluting Coronary Stent System in Subjects With Coronary Artery Lesions), which were published after our meta-analysis, show significant benefit of ultrathin strut drug-eluting stents with a lower rate of target lesion failure, lower rate of target vessel–related myocardial infarction (both early and late), lower rate of ischemia-driven target lesion revascularization, and lower rate of late/very late definite and definite or probable stent thrombosis in comparison with the second-generation thinstrut drug-eluting stents.1 It therefore appears that, despite all the advances in polymer and stent design, the thinness of a strut continues to stand out for potential superior clinical outcomes.5
Journal Title: Circulation
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!