Photo from wikipedia
&NA; We previously described several ionic conductances in human pulmonary fibroblasts, including one activated by two structurally distinct TRPV4 (transient receptor potential, vanilloid‐type, subtype 4)‐channel agonists: 4&agr;PDD (4&agr;‐phorbol‐12,13‐didecanoate) and GSK1016790A.… Click to show full abstract
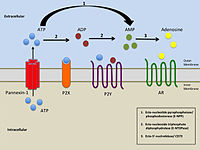
&NA; We previously described several ionic conductances in human pulmonary fibroblasts, including one activated by two structurally distinct TRPV4 (transient receptor potential, vanilloid‐type, subtype 4)‐channel agonists: 4&agr;PDD (4&agr;‐phorbol‐12,13‐didecanoate) and GSK1016790A. However, the TRPV4‐activated current exhibited peculiar properties: it developed slowly over many minutes, exhibited reversal potentials that could vary by tens of millivolts even within a given cell, and was not easily reversed by subsequent addition of two distinct TRPV4‐selective blockers (RN‐1734 and HC‐067047). In this study, we characterized that conductance more carefully. We found that 4&agr;PDD stimulated a delayed release of ATP into the extracellular space, which was reduced by genetic silencing of pannexin expression, and that the 4&agr;PDD‐evoked current could be blocked by apyrase (which rapidly degrades ATP) or by the P2Y purinergic receptor/channel blocker pyridoxalphosphate‐6‐azophenyl‐2′,4′‐disulphonic acid (PPADS), and could be mimicked by exogenous addition of ATP. In addition, we found that the 4&agr;PDD‐evoked current was blocked by pretreatment with RN‐1734 or HC‐067047, by Gd3+ or La3+, or by two distinct blockers of pannexin channels (carbenoxolone and probenecid), but not by a blocker of connexin hemichannels (flufenamic acid). We also found expression of TRPV4‐ and pannexin‐channel proteins. 4&agr;PDD markedly increased calcium flashing in our cells. The latter was abrogated by the P2Y channel blocker PPADS, and the 4&agr;PDD‐evoked current was eliminated by loading the cytosol with 1,2‐bis(o‐aminophenoxy)ethane‐N,N,N′,N′‐tetraacetic acid or by inhibiting Ca2+/calmodulin‐sensitive kinase II using KN93. Altogether, we interpret these findings as suggesting that 4&agr;PDD triggers the release of ATP via pannexin channels, which in turn acts in an autocrine and/or paracrine fashion to stimulate PPADS‐sensitive purinergic receptors on human pulmonary fibroblasts.
Journal Title: American Journal of Respiratory Cell and Molecular Biology
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!