Photo from wikipedia
Cluster headache (CH) has been grouped with other primary headache disorders that share phenotypic manifestations: trigeminal autonomic cephalalgias (TACs) (1,2). By the designation, wallahs accept that patients with cluster headache… Click to show full abstract
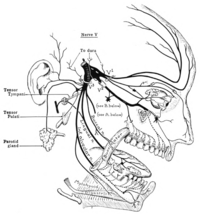
Cluster headache (CH) has been grouped with other primary headache disorders that share phenotypic manifestations: trigeminal autonomic cephalalgias (TACs) (1,2). By the designation, wallahs accept that patients with cluster headache will have cranial autonomic symptoms (CAS), either parasympathetic activation: lacrimation, conjunctival injection, nasal symptoms, aural symptoms, peri-orbital swelling; or sympatholytic manifestations: ptosis, miosis. These tend to be prominent, lateralized, and consistent from attack to attack when compared to migraine (3). Indeed, these symptoms are common in migraine (4) and even manifest in the premonitory phase (5,6). In cluster headache, a crucial question is whether the cranial autonomic symptoms are driven by, or are themselves driving, the pathophysiological process? The trigeminal-autonomic reflex is well understood from an anatomical and physiological perspective (7). The afferent arm of the reflex from the trigeminal nerve passes into the brain through the trigeminal root (8). It is not clear if there is a recurrent afferent that branches prior to the second order synapse in the trigeminocervical complex, or the reflex arises from quintothalamic projections (9,10). The outflow pathway certainly arises in the superior salivatory nucleus (SSN) (11), where there is glutamatergic activation (12). From the SSN, fibres pass via the VIIth cranial (facial) nerve to synapse primarily on classical nicotinic, hexamethonium-sensitive (13,14) post-ganglionic parasympathetic neurons that are nitric oxide synthase (15), vasoactive intestinal polypeptide (VIP) (16) and pituitary adenylate cyclase activating polypeptide (PACAP) (17,18) positive and located in the sphenopalatine ganglion (SPG). VIP (19) and PACAP (20) release can be seen in CH. The pathway from the VIIth nerve produces frequency-dependent (14), neurally-mediated activation of the brain (21) and cranial blood flow (22); with remarkable functional somatotopic fidelity (23). This pathway can be driven from the brainstem (24); with good evidence of projections from the hypothalamus (25,26). Perhaps unsurprisingly given the phenotype (27,28), functional imaging studies have implicated the brain near the most posterior portion of the hypothalamus as being involved in the disorder (29–32). Moreover, deep brain stimulation in this region, being useful for some patients (33–35) and recapitulating some aspects of the condition in patients without the disorder (36), provides further evidence of the importance of this region. While the precise anatomical location of this region remains to be defined, one fundamental issue is whether CH starts from the brain or can be triggered by peripheral nervous system events. On this background, this issue of the Journal has two papers that shed light on the main driver of the pathophysiology in CH. In one study, investigators exploited the known cranial parasympathetic outflow pathway through the SPG in patients implanted with SPG stimulators for the treatment of chronic cluster headache (37). They performed a randomized doubleblind study to compare sham stimulation with low frequency (20Hz) SPG stimulation; high frequency (80–120Hz) stimulation being useful for attacks (38). Interestingly, these limits are consistent with experimental data on the frequency-response characteristics of this pathway (14). Of 20 patients, 16 (80%) had CAS with active and nine (45%) after sham stimulation. Seven of the active group and five of the sham group had a CH attack triggered, with no difference in vasoactive intestinal polypeptide (VIP) or pituitary adenylate cyclase activating polypeptide (PACAP) levels between groups (39). The result suggests peripheral driving of CAS through the efferent arm of the trigeminal-autonomic reflex, the SPG, is not sufficient to initiate CH attacks.
Journal Title: Cephalalgia
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!