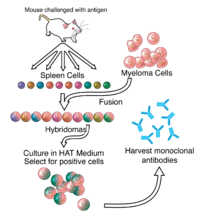
Photo from wikipedia
With advances in molecular engineering and humanization, monoclonal antibodies are one of the fastest-growing classes of biopharmaceuticals. During antibody discovery, antibody from hybridoma or primary B-cell supernatants is screened for… Click to show full abstract
With advances in molecular engineering and humanization, monoclonal antibodies are one of the fastest-growing classes of biopharmaceuticals. During antibody discovery, antibody from hybridoma or primary B-cell supernatants is screened for the desired binding characteristics, and secondary screens measure antibody function and concentration, identify immunoglobulin G (IgG) isotype, and assess cell health. In order to expedite the antibody discovery process, we developed a high-throughput, multiplexed cell and bead-based competition assay that identifies and quantitates mouse IgG isotypes and assesses cell health. No differences in assay performance were observed between single and multiplex formats. The linear range of the assay was from 0.5 to 50 µg/mL, and washing was not required, decreasing assay time and variability. Slight modifications to the protocol allowed quantification of dilute antibody supernatants (0.1–5 µg/mL). Using hybridoma cultures, we showed that cell viability measurements in the assay did not interfere with the bead-based IgG measurements. The assay described here is a simple mix-and-read, no-dilution screen that can reduce the time to antibody cloning and production. The high-content data can differentiate monoclonal and polyclonal wells, determine IgG quantity for downstream functional assays, provide isotype information, and monitor cell proliferation and viability.
Journal Title: SLAS Discovery
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!