Photo from wikipedia
ABSTRACT Oocyte meiotic maturation failure is one of the major causes for female infertility. Meiotic resumption (the G2/M transition) and progression through metaphase I (MI) are two critical stages of… Click to show full abstract
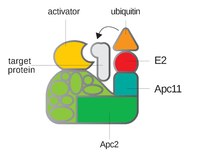
ABSTRACT Oocyte meiotic maturation failure is one of the major causes for female infertility. Meiotic resumption (the G2/M transition) and progression through metaphase I (MI) are two critical stages of oocyte meiotic maturation. Here, we report that centromere protein T (CENP-T), an internal kinetochore protein, plays a critical role in meiotic resumption of mouse oocytes. Depletion of CENP-T by siRNA injection increased the CDH1 (also known as FZR1) level, resulting in increased activity of the anaphase-promoting complex (APC)–CDH1 complex, and further leading to decreased levels of the cyclin protein CCNB1, attenuated maturation-promoting factor (MPF) activity, and finally severely compromised meiotic resumption. The impaired meiotic resumption caused by CENP-T depletion could be rescued by overexpression of exogenous CCNB1 or knockdown of endogenous CDH1. Overexpression of exogenous CENP-T resulted in decreased CDH1 levels, which accelerated the progression of G2/M transition, and accelerated meiotic cell cycle progression after germinal vesicle breakdown (GVBD). Unexpectedly, spindle organization after GVBD was not affected by the overexpression, but the distribution of chromosomes was affected. Our findings reveal a novel role for CENP-T in regulating meiotic progression by acting through CDH1. Highlighted Article: CENP-T plays a critical role in mitosis. Here, we show that CENP-T regulates both meiotic resumption and anaphase entry in meiotic oocytes, which is substantially different from the function of CENP-T in mitosis.
Journal Title: Journal of Cell Science
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!