Photo from wikipedia
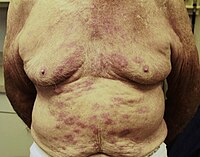
New guidelines have been published by WHO on the diagnosis, treatment, and prevention of leprosy [1]. The recommendations include that all leprosy patients, paucibacillary (PB) and multibacillary (MB), be treated… Click to show full abstract
New guidelines have been published by WHO on the diagnosis, treatment, and prevention of leprosy [1]. The recommendations include that all leprosy patients, paucibacillary (PB) and multibacillary (MB), be treated with three antibacterial drugs. We have examined the evidence used to make these recommendations and find it lacking. The Global Leprosy Programme (GLP) is to be commended for recognising that the absence of evidence-based guidelines for leprosy is a barrier to meeting the challenges outlined in the Global Leprosy Strategy 2016–2020 [2]. The development of guidelines based on the Grading of Recommendations Assessment, Development and Evaluations (GRADE) methodology represents a modern approach to the synthesis of evidence to aid policy makers, programme managers, and healthcare workers. The WHO leprosy guidelines were published after the Guidelines Development Group (GDG), an expert committee, considered the evidence generated by commissioned systematic reviews and formulated guidance. The External Review Group then reviewed the guidance for errors, clarity, and implications but did not review the GDG’s recommendations. Certain recommendations, although agreed upon unanimously by the GDG, are based on insufficient evidence. Guidelines published by WHO carry an authority, which means they are likely to have considerable influence on policy decisions, and it is therefore essential that the recommendations are robust. The WHO Steering Group (SG) developed key questions to be addressed by the guidelines on the role of diagnostic tests, antibacterial chemotherapy, preventive chemotherapy, and vaccines. We agree with the recommendation that no new tests are suitable for diagnosing patients with leprosy. It is very surprising that the SG did not devise any questions concerning leprosy reactions. Leprosy reactions, which are associated with nerve damage and disability, occur in more than 60% of patients with MB leprosy during the first 6 months of treatment [3, 4]. Reactions are difficult to manage and may require prolonged treatment with immunomodulatory agents. The absence of guidance for the effective and safe management of reactions is a major omission. The GLP had an informal consultation on treatment of reactions and prevention of disabilities in leprosy held in Chennai in December 2018 [5]. The guidelines give a conditional recommendation (for which the quality of evidence is rated as low) for changing the antibacterial regimen used to treat patients with PB leprosy. The
Journal Title: PLoS Neglected Tropical Diseases
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!