Photo from wikipedia
In the kidney, purinergic (P2) receptor‐mediated ATP signaling has been shown to be an important local regulator of epithelial sodium transport. Appropriate sodium regulation is crucial for blood pressure (BP)… Click to show full abstract
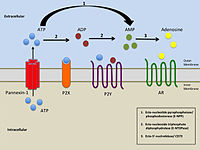
In the kidney, purinergic (P2) receptor‐mediated ATP signaling has been shown to be an important local regulator of epithelial sodium transport. Appropriate sodium regulation is crucial for blood pressure (BP) control and disturbances in sodium balance can lead to hypo‐ or hypertension. Links have already been established between P2 receptor signaling and the development of hypertension, attributed mainly to vascular and/or inflammatory effects. A transgenic mouse model with deletion of the P2X4 receptor (P2X4−/−) is known to have hypertension, which is thought to reflect endothelial dysfunction and impaired nitric oxide (NO) release. However, renal function in this model has not been characterized; moreover, studies in vitro have shown that the P2X4 receptor can regulate renal epithelial Na+ channel (ENaC) activity. Therefore, in the present study we investigated renal function and sodium handling in P2X4−/− mice, focusing on ENaC‐mediated Na+ reabsorption. We confirmed an elevated BP in P2X4−/− mice compared with wild‐type mice, but found that ENaC‐mediated Na+ reabsorption is no different from wild‐type and does not contribute to the raised BP observed in the knockout. However, when P2X4−/− mice were placed on a low sodium diet, BP normalized. Plasma aldosterone concentration tended to increase according to sodium restriction status in both genotypes; in contrast to wild‐types, P2X4−/− mice did not show an increase in functional ENaC activity. Thus, although the increased BP in P2X4−/− mice has been attributed to endothelial dysfunction and impaired NO release, there is also a sodium‐sensitive component.
Journal Title: Physiological Reports
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!