Photo from wikipedia
It has been suggested that the conserved Sgs1-Top3-Rmi1 (STR) helicasedecatenase complex resolves double Holliday junction recombination intermediates (dHJs) as noncrossovers by a process called dissolution. Lichten, Kaur, and GN tested… Click to show full abstract
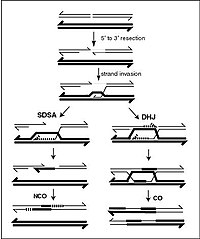
It has been suggested that the conserved Sgs1-Top3-Rmi1 (STR) helicasedecatenase complex resolves double Holliday junction recombination intermediates (dHJs) as noncrossovers by a process called dissolution. Lichten, Kaur, and GN tested this by accumulating dHJs during meiosis... In Saccharomyces cerevisiae, the conserved Sgs1-Top3-Rmi1 helicase-decatenase regulates homologous recombination by limiting accumulation of recombination intermediates that are crossover precursors. In vitro studies have suggested that this may be due to dissolution of double-Holliday junction joint molecules by Sgs1-driven convergent junction migration and Top3-Rmi1 mediated strand decatenation. To ask whether dissolution occurs in vivo, we conditionally depleted Sgs1 and/or Rmi1 during return to growth (RTG), a procedure where recombination intermediates formed during meiosis are resolved when cells resume the mitotic cell cycle. Sgs1 depletion during RTG delayed joint molecule resolution, but, ultimately, most were resolved and cells divided normally. In contrast, Rmi1 depletion resulted in delayed and incomplete joint molecule resolution, and most cells did not divide. rad9∆ mutation restored cell division in Rmi1-depleted cells, indicating that the DNA damage checkpoint caused this cell cycle arrest. Restored cell division in Rmi1-depleted rad9∆ cells frequently produced anucleate cells, consistent with the suggestion that persistent recombination intermediates prevented chromosome segregation. Our findings indicate that Sgs1-Top3-Rmi1 acts in vivo, as it does in vitro, to promote recombination intermediate resolution by dissolution. They also indicate that, in the absence of Top3-Rmi1 activity, unresolved recombination intermediates persist and activate the DNA damage response, which is usually thought to be activated by much earlier DNA damage-associated lesions.
Journal Title: Genetics
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!