Photo from wikipedia
Recent manufacturing problems resulted in a shortage of the only U.S.-licensed yellow fever vaccine. This shortage is expected to lead to a complete depletion of yellow fever vaccine available for… Click to show full abstract
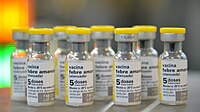
Recent manufacturing problems resulted in a shortage of the only U.S.-licensed yellow fever vaccine. This shortage is expected to lead to a complete depletion of yellow fever vaccine available for the immunization of U.S. travelers by mid-2017. CDC, the Food and Drug Administration (FDA), and Sanofi Pasteur are collaborating to ensure a continuous yellow fever vaccine supply in the United States. As part of this collaboration, Sanofi Pasteur submitted an expanded access investigational new drug (eIND) application to FDA in September 2016 to allow for the importation and use of an alternative yellow fever vaccine manufactured by Sanofi Pasteur France, with safety and efficacy comparable to the U.S.-licensed vaccine; the eIND was accepted by FDA in October 2016. The implementation of this eIND protocol included developing a systematic process for selecting a limited number of clinic sites to provide the vaccine. CDC and Sanofi Pasteur will continue to communicate with the public and other stakeholders, and CDC will provide a list of locations that will be administering the replacement vaccine at a later date.
Journal Title: MMWR. Morbidity and Mortality Weekly Report
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!