Photo from wikipedia
Pure Li4Ti5O12 and Alor C-doped Li4Ti5O12 samples were synthesized by a microwave-assisted solgel method. The structural characterization was performed by X-ray diffraction. The surface morphology was analyzed by scanning electron… Click to show full abstract
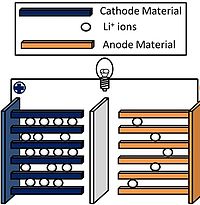
Pure Li4Ti5O12 and Alor C-doped Li4Ti5O12 samples were synthesized by a microwave-assisted solgel method. The structural characterization was performed by X-ray diffraction. The surface morphology was analyzed by scanning electron microscopy. The electrochemical properties were studied by galvanostatic charge and discharge measurements. The results show that after a microwave treatment at 800 °C for 4 h, the size of the particles was 60–70 nm. The crystal structure of Li4Ti5O12 was unchanged and the Ti atom position in the sample was replaced by Al or C, and the dispersion of the doped samples was better than the original sample, which is beneficial for the intercalation and deintercalation of Li + . The measured electrochemical properties indicated that the discharge capacity of the all samples was stable, with the first discharge capacity of the Aland C-doped sample higher than the original sample. The first discharge capacity of Li4Ti5O12/C was 162 mAh/g, which was closer to the theoretical capacity of Li4Ti5O12 (175 mAh/g). After 40 cycle life tests, the discharge capacity could be maintained at 136 mAh/g. The discharge rate capability tests showed that the reversible capacity of Li4Ti5O12/C is restored to 150 mAh/g, with a current density loss of 12 mAh/g compared to the initial discharge capacity, indicating its better structural stability.
Journal Title: International Journal of Electrochemical Science
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!