Photo from wikipedia
Purpose The pharmacology, pharmacokinetics, pharmacodynamics, antimicrobial activity, clinical safety, and current regulatory status of solithromycin are reviewed. Summary Solithromycin is a novel ketolide antibiotic developed for the treatment of community‐acquired… Click to show full abstract
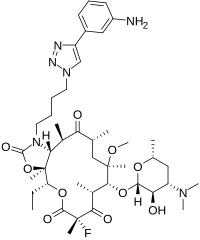
Purpose The pharmacology, pharmacokinetics, pharmacodynamics, antimicrobial activity, clinical safety, and current regulatory status of solithromycin are reviewed. Summary Solithromycin is a novel ketolide antibiotic developed for the treatment of community‐acquired bacterial pneumonia (CABP). Its pharmacologic, pharmacokinetic, and pharmacodynamic properties provide activity against a broad range of intracellular organisms, including retained activity against pathogens displaying various mechanisms of macrolide resistance. Phase III clinical trials of solithromycin demonstrated noninferiority of both oral and i.v.‐to‐oral regimens of 5–7 days’ duration compared with moxifloxacin for patients with moderately severe CABP. Nearly one third of patients receiving i.v. solithromycin experienced infusion‐site reactions. Although no liver‐related adverse events were reported in patients receiving oral solithromycin, more patients receiving i.v.‐to‐oral solithromycin experienced asymptomatic, transient transaminitis, with alanine transaminase levels of >3 to >5 times the upper limit, compared with those treated with moxifloxacin. These results led the Food and Drug Administration to conclude that the solithromycin new drug application was not approvable as filed, adding that the risk of hepatotoxicity had not yet been adequately characterized. The agency further recommended a comparative study of patients with CABP to include approximately 9,000 patients exposed to solithromycin in order to exclude drug‐induced liver injury events occurring at a rate of 1 in 3,000 with 95% probability. Conclusion Solithromycin is a novel ketolide antibiotic with activity against a broad spectrum of intracellular organisms, including those displaying macrolide resistance. While demonstrating noninferiority to a current first‐line agent in the treatment of CABP, concerns for drug‐induced liver injury and infusion‐site reactions have placed its regulatory future in doubt.
Journal Title: American Journal of Health-System Pharmacy
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!