Photo from wikipedia
This work shows that an increase in intracellular ROS through the Rac1–NOX pathway is a general response upon loss of cell–cell adhesion, which is necessary for triggering cell migration. Epithelial… Click to show full abstract
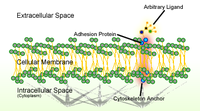
This work shows that an increase in intracellular ROS through the Rac1–NOX pathway is a general response upon loss of cell–cell adhesion, which is necessary for triggering cell migration. Epithelial cells usually trigger their “migratory machinery” upon loss of adhesion to their neighbors. This default is important for both physiological (e.g., wound healing) and pathological (e.g., tumor metastasis) processes. However, the underlying mechanism for such a default remains unclear. In this study, we used the human head and neck squamous cell carcinoma (HNSCC) SAS cells as a model and found that loss of cell–cell adhesion induced reactive oxygen species (ROS) generation and vimentin expression, both of which were required for SAS cell migration upon loss of cell–cell adhesion. We demonstrated that Tiam1-mediated Rac1 activation was responsible for the ROS generation through NADPH-dependent oxidases. Moreover, the ROS–Src–STAT3 signaling pathway that led to vimentin expression was important for SAS cell migration. The activation of ROS, Src, and STAT3 was also detected in tumor biopsies from HNSCC patients. Notably, activated STAT3 was more abundant at the tumor invasive front and correlated with metastatic progression of HNSCC. Together, our results unveil a mechanism of how cells trigger their migration upon loss of cell–cell adhesion and highlight an important role of the ROS–Src–STAT3 signaling pathway in the progression of HNSCC.
Journal Title: Life Science Alliance
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!