Photo from wikipedia
An NAD+-dependent xylitol dehydrogenase from A. flavus (AfXDH) was cloned and successfully expressed in Escherichia coli. AfXDH gene sequence revealed an open reading frame of 1,110 bp, encoding a polypeptide… Click to show full abstract
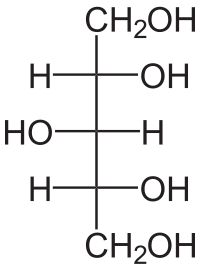
An NAD+-dependent xylitol dehydrogenase from A. flavus (AfXDH) was cloned and successfully expressed in Escherichia coli. AfXDH gene sequence revealed an open reading frame of 1,110 bp, encoding a polypeptide of 369 amino acids with a calculated molecular mass of 38,893 Da. Among various polyols, sorbitol and xylitol were preferred substrates of AfXDH with Km values of 16.2 and 16.9 mM, respectively. AfXDH showed the highest activity in Tris-glycine-NaOH buffer (pH 9.5) at 50°C; it required Zn2+ or Mn2+ for enzyme activity. The half-life at 40°C and half denaturation temperature (T1/2) was 200 min and 45°C, respectively. Bioinformatic analyses along with biochemical properties confirmed that AfXDH belonged to the medium-chain dehydrogenase/reductase family. AfXDH exhibits higher thermostability and k cat values than those of other XDHs. The feasibility of using AfXDH in l-xylulose production was demonstrated. AfXDH, when coupled with Streptococcus pyogenes NADH oxidase, efficiently converted xylitol to l-xylulose with 97% yield, suggesting its usefulness for the industrial l-xylulose production from xylitol.
Journal Title: Frontiers in Bioengineering and Biotechnology
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!