Photo from wikipedia
New hydrophobic derivatives of cinnamic and hydroxycinnamic (ferulic and cumaric) acids obtained by chemical esterification of the carboxylic group with C10 linear alcohol were studied to evaluate their antioxidant capacity… Click to show full abstract
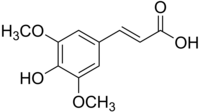
New hydrophobic derivatives of cinnamic and hydroxycinnamic (ferulic and cumaric) acids obtained by chemical esterification of the carboxylic group with C10 linear alcohol were studied to evaluate their antioxidant capacity toward the superoxide anion and hydrogen peroxide in physiological buffer and in extra-virgin olive oil (EVO) or Nigella sativa oils. Results showed that cumaric and ferulic acids have higher antioxidants activity against superoxide anion and hydrogen peroxide than the other compounds. Cumaric acid and its C10-ester derivative were selected to be incorporated into EVO or Nigella sativa oil-based emulsions. The prepared emulsions had a comparable particle size distribution (in the range of 3–4 µm) and physical stability at least up to three months. Nigella sativa oil-based emulsions loaded with cumaric acid or its C10-ester showed a higher capacity to scavenger superoxide anion and hydrogen peroxide than EVO oil-based emulsions. In conclusion, cumaric acid or its C10-ester could promote the antioxidant properties of Nigella sativa oil when formulated as emulsions.
Journal Title: Antioxidants
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!