Photo from wikipedia
During the preparative synthesis of 2-fluorocordycepin from 2-fluoroadenosine and 3′-deoxyinosine catalyzed by E. coli purine nucleoside phosphorylase, a slowdown of the reaction and decrease of yield down to 5% were… Click to show full abstract
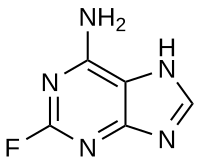
During the preparative synthesis of 2-fluorocordycepin from 2-fluoroadenosine and 3′-deoxyinosine catalyzed by E. coli purine nucleoside phosphorylase, a slowdown of the reaction and decrease of yield down to 5% were encountered. An unknown nucleoside was found in the reaction mixture and its structure was established. This nucleoside is formed from the admixture of 2′,3′-anhydroinosine, a byproduct in the preparation of 3-′deoxyinosine. Moreover, 2′,3′-anhydroinosine forms during radical dehalogenation of 9-(2′,5′-di-O-acetyl-3′-bromo- -3′-deoxyxylofuranosyl)hypoxanthine, a precursor of 3′-deoxyinosine in chemical synthesis. The products of 2′,3′-anhydroinosine hydrolysis inhibit the formation of 1-phospho-3-deoxyribose during the synthesis of 2-fluorocordycepin. The progress of 2′,3′-anhydroinosine hydrolysis was investigated. The reactions were performed in D2O instead of H2O; this allowed accumulating intermediate substances in sufficient quantities. Two intermediates were isolated and their structures were confirmed by mass and NMR spectroscopy. A mechanism of 2′,3′-anhydroinosine hydrolysis in D2O is fully determined for the first time.
Journal Title: Biomolecules
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!