Photo from wikipedia
The agricultural use of organophosphorus pesticides is a widespread practice with significant advantages in crop health and product yield. An undesirable consequence is the contamination of soil and groundwater by… Click to show full abstract
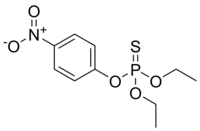
The agricultural use of organophosphorus pesticides is a widespread practice with significant advantages in crop health and product yield. An undesirable consequence is the contamination of soil and groundwater by these neurotoxins resulting from over application and run-off. Here, we design and synthesize the mononuclear zinc(II) complexes, namely, [Zn(AMB)2Cl](ClO4) 1 and [Zn(AMB)2(OH)](ClO4) 2 (AMB = 2-aminomethylbenzimidazole), as artificial catalysts inspired by phosphotriesterase (PTE) for the hydrolysis of organophosphorus compounds (OPs) and simultaneously detect the organophosphate pesticides such as fenitrothion and parathion. Spectral and DFT (B3LYP/Lanl2DZ) calculations revealed that complexes 1 and 2 have a square-pyramidal environment around zinc(II) centers with coordination chromophores of ZnN4Cl and ZnN4O, respectively. Both 1 and 2 were used as a modifier in the construction of a biomimetic sensor for the determination of toxic OPs, fenitrothion and parathion, in phosphate buffer by square wave voltammetry. The hydrolysis of OPs using 1 or 2 generates p-nitrophenol, which is subsequently oxidized at the surface of the modified carbon past electrode. The catalytic activity of 2 was higher than 1, which is attributed to the higher electronegativity of the former. The oxidation peak potentials of p-nitrophenol were obtained at +0.97 V (vs. Ag/AgCl) using cyclic voltammetry (CV) and +0.88 V (vs. Ag/AgCl) using square wave voltammetry. Several parameters were investigated to evaluate the performance of the biomimetic sensor obtained after the incorporation of zinc(II) complex 1 and 2 on a carbon paste electrode (CPE). The calibration curve showed a linear response ranging between 1.0 μM (0.29 ppm) and 5.5 μM (1.6 ppm) for fenitrothion and 1.0 μM (0.28 ppm) and 0.1 μM (0.028 ppm) for parathion with a limit of detection (LOD) of 0.08 μM (0.022 ppm) and 0.51 μM (0.149 ppm) for fenitrothion and parathion, respectively. The obtained results clearly demonstrated that the CPE modified by 1 and 2 has a remarkable electrocatalytic activity towards the hydrolysis of OPs under optimal conditions.
Journal Title: Crystals
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!