Photo from wikipedia
Isothermal titration calorimetry is frequently employed to determine the critical micelle concentration and the micellization enthalpy of surfactants in terms of geometrical characteristics of the titration curves. Previously we have… Click to show full abstract
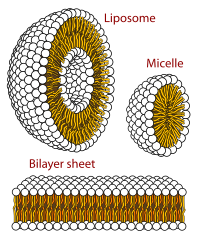
Isothermal titration calorimetry is frequently employed to determine the critical micelle concentration and the micellization enthalpy of surfactants in terms of geometrical characteristics of the titration curves. Previously we have shown theoretically that even for an infinitesimal injection, the heat per titrant mol depends on the stock solution concentration. In this work, we explore experimentally the influence of the stock solution concentration on the geometrical characteristics of the titration curve and its effect in determining the critical micelle concentration and the micellization enthalpy of surfactants. The systematic study of this phenomenology involves a great number of measurements at different temperatures with several repetitions carried out using a robotic calorimeter. As surfactant hexadecyltrimethylamonium bromide was used. The magnitude and shape of the heat titration depend on the stock solution concentration. As a consequence, the inflexion-point, break-point, and step-height decrease until a limiting value. A qualitative analysis suggests that the limiting value depends only on substance. This work shows that graphical methods could not be suitable for the calculation of the critical micelle concentration and micellization enthalpy because the magnitude and shape of the titration curve depend on the stock solution concentration. Micellar properties should be calculated by the application of theoretical models as in the ligand-binding studies.
Journal Title: Entropy
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!