Photo from wikipedia
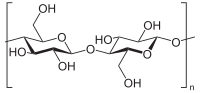
In this study, we chemically modified the short linear glucan (SLG) using the 3-chloro-2-hydroxypropyl trimethylammonium chloride to introduce a positive surface charge via cationization (CSLG). We then prepared CSLG-based binary… Click to show full abstract
In this study, we chemically modified the short linear glucan (SLG) using the 3-chloro-2-hydroxypropyl trimethylammonium chloride to introduce a positive surface charge via cationization (CSLG). We then prepared CSLG-based binary nanocomplex particles through electrostatic interactions with low and high methoxyl pectin. The two new types of binary nanocomplex were comprehensively characterized. It was found that the nanocomplex particles showed a spherical shape with the particle size of <700 nm, smooth surface, homogeneous distribution, and negative surface charge. Fourier transform infrared spectroscopy (FTIR) revealed that the driving forces to form nanocomplex were primarily electrostatic interactions and hydrogen bonding. In addition, increasing the CSLG concentration in the nanocomplex significantly enhanced both thermal stability and digestive stability. By comparing the two complex nanoparticles, the HMP-CSLG has a larger particle size and better stability under the GI condition due to the high content of the methoxy group. Additionally, the HMP-CSLG nanoparticle has a higher encapsulation efficiency and slower release rate under simulated gastrointestinal fluid for tangeretin compared with the LMP-CSLG. These results provide new insights into designing the CSLG-based nanocomplex as a potential oral delivery system for nutraceuticals or active ingredients.
Journal Title: Foods
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!