Photo from wikipedia
The enzymatic degradation of different chitin polymers into chitin oligosaccharides (COSs) is of great significance given their better solubility and various biological applications. Chitinase plays a pivotal role in the… Click to show full abstract
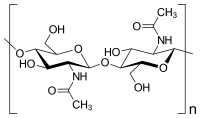
The enzymatic degradation of different chitin polymers into chitin oligosaccharides (COSs) is of great significance given their better solubility and various biological applications. Chitinase plays a pivotal role in the enzymatic preparation of COSs. Herein, a cold-adapted and efficient chitinase (ChiTg) from the marine Trichoderma gamsii R1 was purified and characterized. The optimal temperature of ChiTg was 40 °C, and the relative activity at 5 °C was above 40.1%. Meanwhile, ChiTg was active and stable from pH 4.0 to 7.0. As an endo-type chitinase, ChiTg exhibited the highest activity with colloidal chitin, then with ball-milled and powdery chitin. In addition, ChiTg showed high efficiency when hydrolyzing colloidal chitin at different temperatures, and the end products were mainly composed of COSs with one to three degrees of polymerization. Furthermore, the results of bioinformatics analysis revealed that ChiTg belongs to the GH18 family, and its acidic surface and the flexible structure of its catalytic site may contribute to its high activity in cold conditions. The results of this study provide a cold-active and efficient chitinase and ideas for its application regarding the preparation of COSs from colloidal chitin.
Journal Title: Marine Drugs
Year Published: 2023
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!