Photo from wikipedia
An attractive approach to increase the aqueous apparent solubility of poorly soluble drugs is to formulate them in their amorphous state. In the present study, celecoxib, a poorly soluble drug,… Click to show full abstract
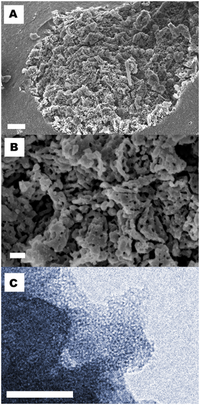
An attractive approach to increase the aqueous apparent solubility of poorly soluble drugs is to formulate them in their amorphous state. In the present study, celecoxib, a poorly soluble drug, was successfully loaded into mesoporous magnesium carbonate (MMC) in its amorphous state via a solvent evaporation method. Crystallization of celecoxib was suppressed, and no reaction with the carrier was detected. The MMC formulation was evaluated in vitro and in vivo in terms of oral bioavailability. Celebra®, a commercially available formulation, was used as a reference. The two celecoxib formulations were orally administrated in male rats (average of n = 6 animals per group), and blood samples for plasma were taken from all animals at different time points after administration. There was no statistical difference (p > 0.05) in AUCinf between the two formulations. The results showed that MMC may be a promising drug delivery excipient for increasing the bioavailability of compounds with solubility-limited absorption.
Journal Title: Molecules
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!