Photo from wikipedia
This study demonstrated the enzymatic hydroxylation of glycitin to 3′-hydroxyglycitin, confirming the structure by mass and nucleic magnetic resonance spectral analyses. The bioactivity assays further revealed that the new compound… Click to show full abstract
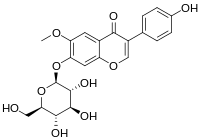
This study demonstrated the enzymatic hydroxylation of glycitin to 3′-hydroxyglycitin, confirming the structure by mass and nucleic magnetic resonance spectral analyses. The bioactivity assays further revealed that the new compound possessed over 100-fold higher 1,1-diphenyl-2-picrylhydrazine free-radical scavenging activity than the original glycitin, although its half-time of stability was 22.3 min. Furthermore, the original glycitin lacked anti-α-glucosidase activity, whereas the low-toxic 3′-hydroxyglycitin displayed a 10-fold higher anti-α-glucosidase activity than acarbose, a standard clinical antidiabetic drug. The inhibition mode of 3′-hydroxyglycitin was noncompetitive, with a Ki value of 0.34 mM. These findings highlight the potential use of the new soy isoflavone 3′-hydroxyglycitin in biotechnology industries in the future.
Journal Title: Plants
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!