Photo from wikipedia
After decades of development, ionic liquid gel polymer electrolytes (ILGPEs) are currently experiencing a renaissance as a promising electrolyte to be used in electrochemical devices. Their inherent tendency towards poor… Click to show full abstract
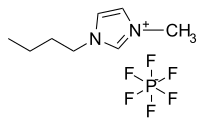
After decades of development, ionic liquid gel polymer electrolytes (ILGPEs) are currently experiencing a renaissance as a promising electrolyte to be used in electrochemical devices. Their inherent tendency towards poor electrochemical properties have limited their applications and commercialization activities. Henceforth, gel polymer electrolyte (GPE) is being introduced to alleviate the abovementioned issues. In this work, the assessment of the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate [BMIM][BF4] in poly (vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) to form ILGPE was done. The relationship of [BMIM][BF4] towards the dielectric properties at different wt. % ratios and temperature was ascertained. The results indicated that [BMIM]BF4 is able to facilitate fast conduction. Moreover, it was found that [BMIM][BF4] could serve as an effective agent in reducing crystallinity and glass transition temperature of the polymer and thus enhanced the ionic conductivity of the samples. Notwithstanding, the ILGPE sample possessed a high thermal stability up to 300 °C and good electrochemical stability of 4.2 V which are beneficial for operation in electrochemical devices. All in all, the correlation between the ionic liquid chemistry and electrochemical performances could provide a valuable insight to rational selection and design for ILGPE electrolytes.
Journal Title: Polymers
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!