Photo from wikipedia
Glycolysis of post-consumer polyethylene terephthalate (PET) waste is a promising chemical recycling technique, back to the monomer, bis(2-hydroxyethyl) terephthalate (BHET). This work presents sodium methoxide (MeONa) as a low-cost catalyst… Click to show full abstract
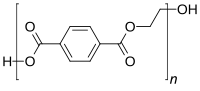
Glycolysis of post-consumer polyethylene terephthalate (PET) waste is a promising chemical recycling technique, back to the monomer, bis(2-hydroxyethyl) terephthalate (BHET). This work presents sodium methoxide (MeONa) as a low-cost catalyst for this purpose. BHET product was confirmed by gas chromatography-mass spectrometry (GCMS), Nuclear Magnetic Resonance (NMR) Spectroscopy, melting point, and Differential Scanning Calorimetry (DSC). It was shown, not surprisingly, that PET conversion increases with the glycolysis temperature. At a fixed temperature of 190 °C, the response surface methodology (RSM) based on the Box-Behnken design was applied. Four independent factors, namely the molar ratio of PET: MeONa (50–150), the molar ratio of ethylene glycol to PET (EG: PET) (3–7), the reaction time (2–6 h), and the particle size (0.25–1 mm) were studied. Based on the experimental results, regression models as a function of significant process factors were obtained and evaluated by analysis of variance (ANOVA), to predict the depolymerization performance of MeONa in terms of PET conversion. Coefficient of determination, R2 of 95% indicated the adequacy for predicted model. Afterward, the regression model was validated and optimized within the design space with a prediction of 87% PET conversion at the optimum conditions demonstrating a deviation of less than 5% from predicted response. A van ‘t Hoff plot confirmed the endothermic nature of the depolymerization reaction. The ceiling temperature (TC = 160 °C) was calculated from Gibbs’ free energy. A kinetic study for the depolymerization reaction was performed and the activation energy for MeONa was estimated from the Arrhenius plot (EA = 130 kJ/mol). The catalytic depolymerization efficiency of MeONa was compared under similar conditions with widely studied zinc acetate and cobalt acetate. This study shows that MeONa’s performance, as a glycolysis catalyst is promising; in addition, it is much cheaper and environmentally more benign than heavy metal salts. These findings make a valuable contribution towards the chemical recycling of post-consumer PET waste to meet future recycling demands of a circular economy.
Journal Title: Polymers
Year Published: 2023
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!