Photo from wikipedia
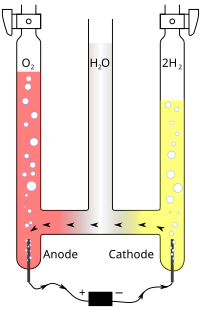
The electrochemical chlorate reduction at the Pt electrode in 0.5 M H2SO4 deaerated solutions has been studied using potentiostatic steady-state voltammetry. The kinetics parameters (Tafel slope, charge transfer coefficient, current… Click to show full abstract
The electrochemical chlorate reduction at the Pt electrode in 0.5 M H2SO4 deaerated solutions has been studied using potentiostatic steady-state voltammetry. The kinetics parameters (Tafel slope, charge transfer coefficient, current density, and reaction order) were evaluated in function of chlorate concentration (1x10-4 � 0.2 M KClO3). The process of chlorate reduction is a complex one that implies two charge transfer controlled steps with formation of free radicals and an extent potential region controlled by the concentration polarization. The current density dependence of chlorate concentration tends to an exponential growth at concentration � 0.1 M KClO3 and becomes exponential in the conditions of the catalyst system presence. In the second charge transfer, a surface reaction between free radical �Cl-2 and platinum electrode with formation of complex anions PtCL42- and PtCL62- is responsible for the rapid increase of the reaction rate.
Journal Title: Revista de Chimie
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!