Photo from wikipedia
AIM Midazolam is a commonly used marker substrate for the in vivo assessment of CYP3A activity. Reliable pharmacokinetic assessment at sub-pharmacological doses of midazolam requires an ultra-sensitive analytical method. METHODS… Click to show full abstract
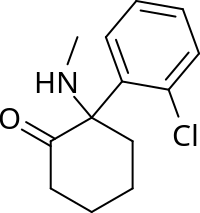
AIM Midazolam is a commonly used marker substrate for the in vivo assessment of CYP3A activity. Reliable pharmacokinetic assessment at sub-pharmacological doses of midazolam requires an ultra-sensitive analytical method. METHODS A new, ultra-sensitive LC-MS/MS method for the determination of midazolam in human plasma using SPE was developed and fully validated. The lowest limit of quantitation is 0.1 pg/ml with a sample volume of 500 μl. RESULTS/CONCLUSION The following parameters were validated: sensitivity, assay accuracy and precision, linearity, selectivity, and stability of midazolam at pertinent analytical and storage conditions. The validated method was utilized successfully for the sample assay during a midazolam microdosing study for the evaluation of CYP3A4 activity of a clinical candidate.
Journal Title: Bioanalysis
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!