Photo from wikipedia
Uveitis is a major cause of ocular morbidity, potentially leading to significant visual impairment. The recent adoption of alternative drug delivery options has led to the development of new sustained-delivery… Click to show full abstract
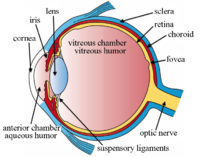
Uveitis is a major cause of ocular morbidity, potentially leading to significant visual impairment. The recent adoption of alternative drug delivery options has led to the development of new sustained-delivery corticosteroid systems, able to manage successfully chronic noninfectious posterior uveitis. The treatment goal is to target the site of inflammation with low dose of corticosteroids, delivered over an extended period of time, to minimize the cumulative damage resulting from repeated recurrences, reducing both injections frequency and ocular side effects. This article will review the pharmacology and preliminary clinical data of the 0.18 mg fluocinolone acetonide intravitreal implant (YUTIQ™), to show its efficacy and safety in the treatment of noninfectious posterior uveitis.
Journal Title: Therapeutic delivery
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!