Photo from wikipedia
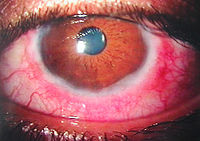
Objectives To assess the safety of dermatological 0.1% tacrolimus ointment when used topically and its efficacy in the treatment of vernal keratoconvinctivtis. METHODS The quasi-experimental, multi-centre study was conducted at… Click to show full abstract
Objectives To assess the safety of dermatological 0.1% tacrolimus ointment when used topically and its efficacy in the treatment of vernal keratoconvinctivtis. METHODS The quasi-experimental, multi-centre study was conducted at the Gujranwala Medical College/District Headquarters Teaching Hospital, Gujranwala, and the Gomal Medial College/Mufti Mehmood Teaching Hospital, Dera Ismail Khan, Pakistan, from July 2019 to March 2020, and comprised patients of severe vernal keratoconvinctivtis. Symptoms and clinical signs were graded on a pre-devised scale. Patients were given small amount of tacrolimus 0.1% ointment applied to the inferior conjunctival fornix before going to bed. The duration of treatment was 3 months and the patients were followed up for up to 6 months. Data was analysed using SPSS 20. RESULTS Of the 50 patients, 30(60%) were males and 20(40%) were females. The overall mean age was 10.64±3.199 years. Mean symptom score and clinical signs score gradually reduced on each follow-up (p<0.05). Mild recurrence was noted in 12(24%) patients who were managed with lubricants and anti-histamine topical drops. No complication was noted. CONCLUSIONS Tacrolimus 0.1% was found to be effective and safe in the treatment of severe refractory vernal keratoconvinctivtis even when given once a day. Clinical Trial Registration Chinese Clinical Trial Registry Id: ChiCTR2000031929 link: www.chictr.org.cn/hvshowproject.aspx?id=28053.
Journal Title: JPMA. The Journal of the Pakistan Medical Association
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!