
Catalyst-Promoted Diastereodivergent Enantioselective Formal oxa-Diels-Alder Reaction of Unsaturated Ketones with Enoates Under Liquid-Assisted Grinding Conditions.
Sign Up to like & getrecommendations! Published in 2022 at "ChemSusChem"
DOI: 10.1002/cssc.202200028
Abstract: Chiral heterocycles are widely occurring in many compounds of interest, but their efficient synthesis is challenging. We present here the enantioselective and diastereoselective synthesis of densely substituted chiral pyran derivatives. Diastereodivergency of the oxa-Diels-Alder reaction… read more here.
Keywords: oxa diels; reaction; liquid assisted; diels alder ... See more keywords

Supramolecular Phthalimide Networks via Tandem Diels-Alder Reaction-Aromatization Using Biomass-derived Furanic Dienes.
Sign Up to like & getrecommendations! Published in 2022 at "Macromolecular rapid communications"
DOI: 10.1002/marc.202200711
Abstract: The design and synthesis of phthalimide derivatives are important goals for applications in fields like pharmaceutical science and optoelectronics. In the present study, we devised a facile and convenient synthetic pathway (no heat or acid/catalyst… read more here.
Keywords: biomass derived; diels alder; aromatization; alder reaction ... See more keywords

Temperature-controlled cross-linking of silver nanoparticles with diels-alder reaction and its application on antibacterial property
Sign Up to like & getrecommendations! Published in 2017 at "Applied Surface Science"
DOI: 10.1016/j.apsusc.2017.01.162
Abstract: Abstract Sliver nanoparticles (AgNPs) were synthesized and functionalized with furan group on their surface, followed by the reverse Diels-Alder (DA) reaction with bismaleimide to vary the particle size, so as to give different antibacterial activities.… read more here.
Keywords: diels alder; size; reaction; cross linking ... See more keywords

Synthesis of 5-vinyl-2-norbornene through Diels-Alder reaction of cyclopentadiene with 1, 3-butadiene in supercritical carbon dioxide
Sign Up to like & getrecommendations! Published in 2017 at "Chinese Chemical Letters"
DOI: 10.1016/j.cclet.2016.12.018
Abstract: Abstract An efficient method for the synthesis of 5-vinyl-2-norbornene from cyclopentadiene and 1,3-butadiene was developed. The Diels–Alder reaction of cyclopentadiene with 1,3-butadiene proceeded smoothly in supercritical carbon dioxide in the absence of any polymerization inhibitor… read more here.
Keywords: diels alder; synthesis vinyl; cyclopentadiene butadiene; vinyl norbornene ... See more keywords

β-Trifluoromethylated nitroethenes in Diels-Alder reaction with cyclopentadiene: A DFT computational study
Sign Up to like & getrecommendations! Published in 2018 at "Journal of Fluorine Chemistry"
DOI: 10.1016/j.jfluchem.2017.12.008
Abstract: Abstract Mechanistic aspects of reactions between β-trifluoromethylated nitroethenes and cyclopentadiene has been examined using DFT computational methods It was found, that all considered processes have a polar nature. This polar character is given by the… read more here.
Keywords: nitroethenes diels; diels alder; trifluoromethylated nitroethenes; alder reaction ... See more keywords

NIR induced self-healing electrical conductivity polyurethane/graphene nanocomposites based on Diels−Alder reaction
Sign Up to like & getrecommendations! Published in 2018 at "Polymer"
DOI: 10.1016/j.polymer.2018.02.036
Abstract: Abstract In this work, a crosslinker terminal maleimide groups (EDM) has been synthesized and was used in reactions with the pendant furan groups of polyurethane (PUE) to prepare the self-healing electrically conductive composites based on… read more here.
Keywords: induced self; diels alder; based diels; graphene ... See more keywords

A self-healing and recyclable polyurethane/halloysite nanocomposite based on thermoreversible Diels-Alder reaction
Sign Up to like & getrecommendations! Published in 2020 at "Polymer"
DOI: 10.1016/j.polymer.2020.122894
Abstract: Abstract The realization of recyclable materials is an urgent problem to be solved for extending practical applications in industry. Herein, a simple method was proposed to prepared the recyclable materials thorough dynamic reversible chemistry. In… read more here.
Keywords: diels alder; based thermoreversible; polyurethane; self healing ... See more keywords
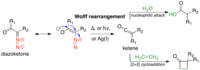
Synthesis of the hexacyclic triterpene core of the jujuboside saponins via tandem Wolff rearrangement-intramolecular ketene hetero-Diels-Alder reaction.
Sign Up to like & getrecommendations! Published in 2018 at "Tetrahedron"
DOI: 10.1016/j.tet.2018.04.051
Abstract: The jujubosides are saponin natural products reported to have immunoadjuvant, anticancer, antibacterial, antifungal, and antisweet activities. The triterpene component, jujubogenin contains a unique tricyclic ketal motif comprising the DEF ring system. Herein, we describe our… read more here.
Keywords: diels alder; wolff rearrangement; tandem wolff; rearrangement intramolecular ... See more keywords

Synthetic studies toward lindenane-type dimers via Diels-Alder reaction
Sign Up to like & getrecommendations! Published in 2018 at "Tetrahedron"
DOI: 10.1016/j.tet.2018.10.005
Abstract: Abstract Lindenane-type dimers are natural sesquiterpenoids, mainly from Chloranthaceae, possessing an unique heptacyclic skeleton and diverse bioactivities. Inspection of their structures suggested the possibility of deploying an endo Diels-Alder reaction to construct the key cyclohexene… read more here.
Keywords: diels alder; lindenane type; type dimers; lindenane ... See more keywords

Diastereoselective synthesis of highly substituted tetrahydroquinolines using benzoylacetonitrile via aza Diels–Alder reaction
Sign Up to like & getrecommendations! Published in 2021 at "Tetrahedron Letters"
DOI: 10.1016/j.tetlet.2021.153063
Abstract: Abstract Diastereoselective aza-Diels–Alder reaction between in situ generated 2-aminophenyl-substituted enones and α-cyano-α,β-unsaturated aromatic ketone has been developed. In the presence of TEA as a catalyst prepared highly substituted tetrahydroquinolines with an all-carbon quaternary center in… read more here.
Keywords: aza diels; alder reaction; highly substituted; diels alder ... See more keywords

Tuning the Diels-Alder Reaction for Bioconjugation to Maleimide Drug-Linkers.
Sign Up to like & getrecommendations! Published in 2018 at "Bioconjugate chemistry"
DOI: 10.1021/acs.bioconjchem.8b00320
Abstract: The thiol-maleimide linkage is widely used for antibody-drug conjugate (ADC) production; however, conjugation of maleimide-drugs could be improved by simplified procedures and reliable conjugate stability. Here, we report the evaluation of electron-rich and cyclic dienes… read more here.
Keywords: diels alder; reaction bioconjugation; drug; reaction ... See more keywords