
Anifrolumab: First Approval
Sign Up to like & getrecommendations! Published in 2021 at "Drugs"
DOI: 10.1007/s40265-021-01604-z
Abstract: Anifrolumab (anifrolumab-fnia; Saphnelo™) is a monoclonal antibody antagonist of the type 1 interferon receptor (IFNAR). It is being developed by AstraZeneca (under license from Medarex, now Bristol-Myers Squibb) for the treatment of autoimmune disorders, including… read more here.
Keywords: sle; first approval; treatment; anifrolumab first ... See more keywords

An evaluation of anifrolumab for use in adults with systemic lupus erythematosus
Sign Up to like & getrecommendations! Published in 2022 at "Expert Review of Clinical Immunology"
DOI: 10.1080/1744666x.2022.2123793
Abstract: ABSTRACT Introduction Type 1 interferons play a key role in the pathogenesis of systemic lupus erythematosus (SLE). An important clinical question is whether inhibiting the type 1 interferon pathway reduce the disease activity in SLE… read more here.
Keywords: anifrolumab; sle patients; systemic lupus; type ... See more keywords
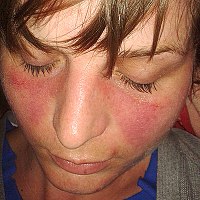
Anifrolumab for treatment of refractory cutaneous lupus erythematosus
Sign Up to like & getrecommendations! Published in 2022 at "Clinical and Experimental Dermatology"
DOI: 10.1111/ced.15335
Abstract: Cutaneous lupus erythematosus (CLE) is a spectrum of skin changes related to systemic lupus erythematosus (SLE), a family of autoimmunity manifesting characteristic multisystem inflammation and damage. Treatment of CLE continues to evolve, especially for patients… read more here.
Keywords: cle; treatment; lupus erythematosus; anifrolumab ... See more keywords

OP0001 The lupus low disease activity state (LLDAS) definition discriminates responders in a systemic lupus erythematosus (SLE) trial: post-hoc analysis of the phase iib muse trial of anifrolumab
Sign Up to like & getrecommendations! Published in 2017 at "Annals of the Rheumatic Diseases"
DOI: 10.1136/annrheumdis-2017-eular.2464
Abstract: Background A LLDAS definition has received preliminarily validation. Achieving low disease activity by this definition is associated with protection from damage accrual for patients (pts) with SLE.1 However, it has not been evaluated as an… read more here.
Keywords: definition; trial; muse; placebo ... See more keywords

POS0683 NOVEL STRINGENT OUTCOME MEASURES APPLIED TO THE PHASE 2 AND 3 ANIFROLUMAB TRIALS
Sign Up to like & getrecommendations! Published in 2021 at "Annals of the Rheumatic Diseases"
DOI: 10.1136/annrheumdis-2021-eular.702
Abstract: Patients with systemic lupus erythematosus (SLE) who received anifrolumab, a type I interferon receptor antibody, had greater BILAG–based Composite Lupus Assessment (BICLA) response rates vs placebo at Week (W)52 in the phase 2 MUSE1 and… read more here.
Keywords: response; placebo; bicla; astrazeneca ... See more keywords