
Biomarker Qualification at the European Medicines Agency: A Review of Biomarker Qualification Procedures From 2008 to 2020
Sign Up to like & getrecommendations! Published in 2022 at "Clinical Pharmacology and Therapeutics"
DOI: 10.1002/cpt.2554
Abstract: Regulatory qualification of biomarkers facilitates their harmonized use across drug developers, enabling more personalized medicine. This study reviews various aspects of the European Medicines Agency’s (EMA’s) biomarker qualification procedure, including frequency and outcome, common challenges,… read more here.
Keywords: qualification procedures; european medicines; biomarker; qualification ... See more keywords

Post‐Marketing Requirements for Cancer Drugs Approved by the European Medicines Agency, 2004–2014
Sign Up to like & getrecommendations! Published in 2022 at "Clinical Pharmacology and Therapeutics"
DOI: 10.1002/cpt.2679
Abstract: To address unresolved questions about drug safety and efficacy at the time of approval, the European Medicines Agency (EMA) may require that manufacturers conduct additional studies during the postmarketing period. As a growing proportion of… read more here.
Keywords: medicines agency; european medicines; 2004 2014; cancer drugs ... See more keywords

Safety monitoring of COVID‐19 vaccines: perspective from the European Medicines Agency
Sign Up to like & getrecommendations! Published in 2022 at "Clinical Pharmacology and Therapeutics"
DOI: 10.1002/cpt.2828
Abstract: Prior to deployment of coronavirus disease 2019 (COVID‐19) vaccines in the European Union in 2021, a high vaccine uptake leading to an unprecedented volume of safety data from spontaneous reports and real‐world evidence, was anticipated.… read more here.
Keywords: medicines agency; safety; european medicines; covid vaccines ... See more keywords

Mobility endpoints in marketing authorisation of drugs: what gets the European medicines agency moving?
Sign Up to like & getrecommendations! Published in 2022 at "Age and Ageing"
DOI: 10.1093/ageing/afab242
Abstract: Abstract Background Mobility is defined as the ability to independently move around the environment and is a key contributor to quality of life, especially in older age. The aim of this study was to evaluate… read more here.
Keywords: medicines agency; european medicines; mobility; authorisation drugs ... See more keywords

Analysis of the Gene Therapies Authorized by the United States Food and Drug Administration and the European Medicines Agency.
Sign Up to like & getrecommendations! Published in 2023 at "Medical care"
DOI: 10.1097/mlr.0000000000001840
Abstract: Background: Gene therapy, altering the genes inside human cells, has recently emerged as an alternative for preventing and treating disease. Concerns have been expressed about the clinical value and the high cost of gene therapies.… read more here.
Keywords: european medicines; food drug; gene therapies; drug administration ... See more keywords

Food and Drug Administration vs European Medicines Agency: Review times and clinical evidence on novel drugs at the time of approval
Sign Up to like & getrecommendations! Published in 2019 at "British Journal of Clinical Pharmacology"
DOI: 10.1111/bcp.14130
Abstract: The Food and Drug Administration (FDA) and European Medicines Agency (EMA) now have expedited review procedures for new drugs. We compared the review times of medicines licensed by the 2 agencies and explored differences in… read more here.
Keywords: drug administration; time; review; european medicines ... See more keywords

Use of real-world evidence in postmarketing medicines regulation in the European Union: a systematic assessment of European Medicines Agency referrals 2013–2017
Sign Up to like & getrecommendations! Published in 2019 at "BMJ Open"
DOI: 10.1136/bmjopen-2018-028133
Abstract: Objectives To assess the use, and evaluate the usefulness, of non-interventional studies and routinely collected healthcare data in postmarketing assessments conducted by the European Medicines Agency (EMA). Design We reviewed and systematically assessed all referrals… read more here.
Keywords: non interventional; european medicines; product; evidence ... See more keywords

Clinical study reports published by the European Medicines Agency 2016–2018: a cross-sectional analysis
Sign Up to like & getrecommendations! Published in 2023 at "BMJ Open"
DOI: 10.1136/bmjopen-2022-068981
Abstract: Objectives To describe the characteristics of clinical study report (CSR) documents published by the European Medicines Agency (EMA), and for included pivotal trials, to quantify the timeliness of access to trial results from CSRs compared… read more here.
Keywords: medicines agency; published european; european medicines; clinical study ... See more keywords

Response by Bernstein to Letter Regarding Article, "Anthracycline Cardiotoxicity: Worrisome Enough to Have You Quaking?"
Sign Up to like & getrecommendations! Published in 2018 at "Circulation Research"
DOI: 10.1161/circresaha.118.312921
Abstract: We thank Dr Lipshultz for pointing out an error in our characterization of the European Medicines Agency action on the use of dexrazoxane as a cardioprotective agent for patients receiving anthracycline chemotherapy.1 One of the… read more here.
Keywords: european medicines; medicines agency; cardiotoxicity; letter ... See more keywords
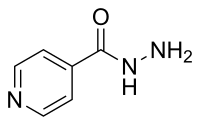
Optimization and Validation of a Chromatographic Method for the Quantification of Isoniazid in Urine of Tuberculosis Patients According to the European Medicines Agency Guideline
Sign Up to like & getrecommendations! Published in 2018 at "Antibiotics"
DOI: 10.3390/antibiotics7040107
Abstract: Isoniazid is a drug that is widely used against tuberculosis. However, it shows high interpatient variability in metabolism kinetics and clinical effect, which complicates the prescription of the medication and jeopardizes the success of the… read more here.
Keywords: european medicines; isoniazid urine; method; medicines agency ... See more keywords

Focus on Clozapine Withdrawal- and Misuse-Related Cases as Reported to the European Medicines Agency (EMA) Pharmacovigilance Database
Sign Up to like & getrecommendations! Published in 2020 at "Brain Sciences"
DOI: 10.3390/brainsci10020105
Abstract: Background: Clozapine is of high clinical relevance for the management of both treatment-resistant schizophrenia and psychotic disturbances with concurrent drug misuse. Although the molecule presents with a range of well-known side-effects, its discontinuation/withdrawal syndrome has… read more here.
Keywords: european medicines; clozapine; misuse; medicines agency ... See more keywords